- Standards, Guidelines
The New EU GMP Annex 1 Draft
Impact on Environmental Monitoring Programs
Introduction
On December 20th, after intensive research and debates, the draft for a Revision of ANNEX 1 ("Manufacture of Sterile Medicinal Products") of the EU Guideline for GOOD MANUFACTURING PRACTICE for drug products and drug substances was published for public comment. This updated document will set a milestone for adjustments needed within European agencies overseeing drug products regulatory applications.
During the creation process, the US FDA and PIC/S were consulted by and partnered with the EU on the proposal, showing the critical need to have standardized regulations reflecting the actual state of sterile pharmaceutical manufacturing.
This document provides a summary of the new aspects of environmental monitoring in sterile manufacturing and its potential implications to the Pharmaceutical industry.
Annex 1 Structure
The new structure provides a comprehensive understanding of where to find relevant content. While the former 2008 version was not well organized and became a patchwork of changes over time, the new authors compiled the content in a logical, easy-to-follow way.
Quality and Manufacturing Officers interested in environmental monitoring will readily find relevant content primarily in Chapter 5 “Premises” and Chapter 9 “Viable and non-viable environmental and process monitoring”.
At 50 pages in total, the content is much more detailed compared to the 16 pages of the previous version. Simple word searches show the importance of certain aspects in comparison to the 2008 version. For example, the word “risk” can be found 92 times compared to only 20 times before. Similarly, “microbial” is now present 68 times compared to 23 counts previously. One could easily identify that the concepts of the ICH guidelines Q8, Q9 and Q10 have found significant representation in this new document.
Cleanroom Classification and Qualification
In the Annex 1 draft, most of the relevant principles of the ISO 14644-1:2015 standard are included (Chapter 5.26). The initial number of sampling sites, their even distribution and related sampling volume in critical zones (Grade A and B) rely on this standard.
However, it is clearly stated that these are only the minimum requirements sufficient to classify a cleanroom. All further decisions must be based on process knowledge and risk assessment. Consequently, it will become very difficult in the future to defend why lesser parameters for the qualification were chosen, especially for inspection of manufacturing environment sampling. This ties deeply with the statement in Chapter 5.28, where “Clean room qualification (including classification) should be clearly differentiated from operational process environmental monitoring”. A clear differentiation needs to be made between each phase of a clean environment's lifetime.
Let’s bring this concept into a simple equation:
INITIAL CLASSIFICATION ≠ RE-QUALIFICATION ≠ PROCESS MONITORING
The classification of a cleanroom, covered in the ISO 14644-1:2015 standard, is based on particle load. There are no microbial limits given for this part of the process, but there has been a major change towards the 2008 version of the guideline:
In chapter 5.25, particles of the size equal to or bigger than 5 μm have been removed from the classification and qualification limit table for Grade A but kept in the recommended limits for monitoring of the process environment.
The reasons for the de-emphasis on the 5 μm limit in Grade A class includes:
- Harmonization of the European Requirements with the recent release of ISO 14644-1, where the 5 μm limit in ISO Class 5 has already been removed.
- Sampling and statistical limitations for particles in low concentrations make this classification inappropriate.
- Sample collection limitations for both particles in low concentrations and sizes greater than 1 μm make classification at this particle size inappropriate, due to potential particle losses in the sampling system.
De-emphasis of the 5 μm limit only refers to the cleanroom classification process. The 5 μm particles still represent an important indicator of possible contamination during the manufacturing process and, therefore, must be kept under control continuously during filling and manufacturing.
Discrepancy in the treatment of 5 μm particles between classification and monitoring are of foremost concern and may cause discussion on the possible risk of not considering certain particle sizes during initial qualification. These sizes will need to be within certain limits during monitoring.
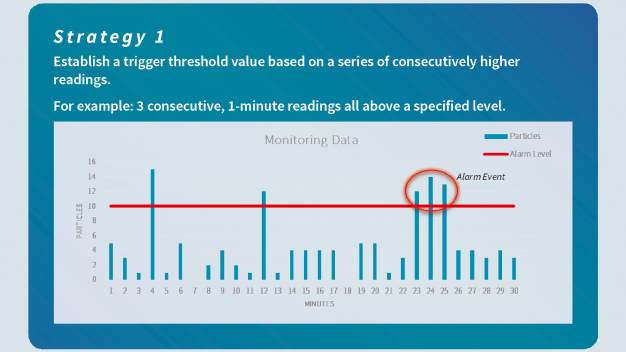
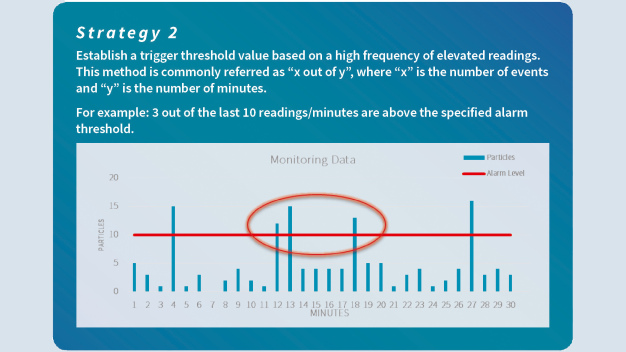
The language pertaining to the responsibility of defining alert and action levels and limits has been made stronger and clearly refers to the cleanroom user, who must define the appropriate values based on a formal risk assessment and data trending analysis. This change emphasizes the expectation of regulators that manufacturers set their action and alert limits based on historical data, process knowledge and a risk-based approach. In addition, it is important not only to define particle limits, but also an appropriate alarm strategy which encourages the evaluation of ISO 14644-2 and its recommended practices (paragraph B.3.4).
The following strategies consider the importance of evaluating an alert or alarm situation using a series of events rather than a single spot valu (Abb. 1 + Abb. 2)
Requalification Frequency
Chapter 5.29 gives manufacturers a challenge: Bi-annual requalification of critical zones (Grade A and B) are becoming a standard of the industry. It is already a widespread practice, but many pharmaceutical companies have differing strategies that will need to be thoroughly explained in upcoming inspections. Modern technologies, including real-time methods for viable counts, that minimize downtimes caused by the requalification process and increase productivity will become more crucial to the success of pharmaceutical companies.
NOTE Throughout the new draft, Grade A and B environments are considered almost equal in the way they are treated for cleanliness.
Annex 1 and Microbial Impurities
Microbial impurities can be divided into “viable” and “non-viable” particles. “Non-viable” impurities are inert and will not contain any microorganisms, and readers of the new regulation should be educated on all technical terms used to prevent misunderstandings of the document.
Laser-based particle counting, typically used for determining “non-viable” levels in a critical pharmaceutical environment, displays both “non-viable” and "viable" particles, which is comprised of "viable and culturable" and “viable but not culturable” (VBNC) impurities. The following equation represents what is seen by laser-based particle counting:
NON-VIABLE = INERT
+ VIABLE AND CULTURABLE
+ VIABLE BUT NOT CULTURABLE
This equation is nonsense but its components have widespread usage, and therefore difficult to change. At a minimum, people using this terminology should be aware of the potential drawbacks from the habitual use of this language.
Cleanrooms for pharmaceutical use are not classified by microbiological parameters, but rather on the non-viable/inert aspect. Therefore, microbiological considerations start when the rooms are qualified for intended use. As in the 2008 version, this is called the “in operation” stage and proposed action limits can be found in Table 2 of the draft. Although the values in the table look familiar, some major changes have been made:
- The values for Grade A zones are now set to 1 and not <1.
- No averaging of results is allowed.
Consequently, manufacturers are no longer allowed to “average out” non-welcome results by looking at a scale of multiple measurements.
Each single result should be considered and cause a deviation resulting in a full investigation. However, this is only true for the qualification of the “in operation” stage and does not apply for the monitoring of the process. True routine “in operation” monitoring limits can only be based on historical data and locations, frequency, volume and duration of monitoring on a risk-based approach and data generated during the qualification, as stated in Chapter 9.5. This may create some confusion between the qualification stage's “in operation” and the routine monitoring program's “in operation”.
Chapters 9.7 and 9.27 follow previously established standards in terms of viable sampling which can be found in other regulatory documents. In essence, sampling should be done as close as possible to the critical area in Grade A environments, but without posing any risk to the process and sampling itself. To do both has been a long-lasting dilemma, and often requires a specialized approach using technologies such as single use.
The frequency of viable sampling has received an almost revolutionary renewal in the Annex 1 draft and has become integrated with increasing control over the process by scientifically sound rationale. Chapter 9.25 indicates that sampling must be frequent can be performed using a combination of methods, leaving the decision to the manufacturer as to which sort of methodology and resulting data should be considered as relevant for the sampling point. As always, the reasoning for all decisions must be documented and based on risk assessment and historical/scientific data. Interestingly these strategies also apply to personnel monitoring (Chapter 9.26). At the moment, manufacturers tend to avoid multiple samplings of operators in order to prevent contamination build-up and the subsequent risk to the process and products. A possible solution could be the implementation of more sampling techniques that are not susceptible for residuals, such as the use of swabs instead of contact plates.
A greater emphasis on the qualification and monitoring of personnel is seen in Chapter 4.4. The stricter requirements may result in operators taking longer to contribute to the productivity of the company (until they are fully qualified). Good personnel will become an even more precious resource in the manufacturing of sterile drugs.
One significant change is that viable sampling should be performed continuously during routine process monitoring, as stated in Chapter 9.27. It will no longer be acceptable to have only small, snapshot sampling that does not characterize the entire manufacturing process. This concept was applied in the 2008 version for “non-viable” counts and has now been expanded into “viable” counts, creating some short-term challenges for manufacturers. Continuous data generation can only be achieved by either real-time methods or long-term, traditional viable sampling that is quasi-continuous. The right combination of methods will become critical in the decision-making process, and it will be an area of interest to see how inspectors push these requirements into the field and how manufacturers will respond.
Grade A and B zones are now considered almost equivalent in how they are treated from a monitoring perspective, and Chapter 9.33 imposes on manufacturers the need to identify all microorganisms found in these environments down to the species level. This new requirement emphasizes:
- The importance of Grade B in final product quality.
- The need for investigations in both cleanrooms.
- The need for understanding the instruments used in these zones and their capability to contribute to contamination.
Trending of environmental data, which has already been implemented on a worldwide scale, has finally found its representation in Chapter 9.33.
Conclusion
The consultation document for the new Annex 1 revision provides insight into upcoming regulatory trends. In terms of environmental monitoring content, there is significant emphasis placed on manufacturers basing their decisions on scientifically sound and historical data while applying a risk-based approach.
From a microbiology and particle contamination perspective, this document may push modern, relevant and scientifically sound monitoring methods into the pharmaceutical world, in addition to a reasonable trending approach to data analysis.
The overall quality of products is sure to increase with the released draft, and a stronger and deeper understanding of cleanroom performance inside each single company should be fostered by allowing continuing discussion and evaluation of the collected sampling data, and development of user set alert and action levels. Monitoring plans should be proactively revised using growing knowledge of the process and risk assessment tools. Rather than considering each single monitoring session as isolated from the whole program, consider it part of a bigger picture.
References
- Annex 1 draft version 2017, "Manufacture of Sterile Medicinal Products”. EU GMP Guide for Good manufacturing practice for drug products and drug substances.
- Eudralex Volume 4 – Annex 1 2008
- ISO 14644-1: 2015 – Cleanroom Classification Standard
- ISO 14644-2:2015 – Cleanroom Monitoring Standard
Disclaimer
The main goal of this paper is to summarize comments and express the authors' position about the main changes included in the consultation version of the new Annex 1 from December 20th, 2017. The authors are fully aware that this document is not in its final stage and only represents an intermediate milestone towards the final version. Nonetheless, it is considered that the content of the draft displays the main stream of the opinion from the regulatory position in Europe.
Authors
Daniele Pandolfi
Product Line Manager Data management/Aerosol
Particle Measuring Systems
Daniele Pandolfi has had experience in particle counter instrumentation and cleanroom contamination control for over ten years. While building strong customer relationships, he has helped many people solve their cGMP issues. Outside of work, he is a semi-professional photographer, an enthusiast of electronics, a lover of new technology, and a keen traveler.
Frank Panofen, PhD
Director and General Manager Life Sciences
Particle Measuring Systems
Dr. Panofen has a Diploma in Chemistry from the University of Bielefeld and a PhD in molecular and cell biology from the University of Osnabrück. He has expansive experience in the field of applied pharmaceutical microbiology.
Frank has been an invited speaker at international conferences including ECA and PDA, with a strong regulatory background in pharmaceuticals. He is a certified Microbiological Laboratory Manager from ECA.
![]()
Particle Measuring Systems Germany GmbH
Im Tiefen See 45
64293 Darmstadt
Germany
Phone: +49 351 88963850
email: PMSGermany@pmeasuring.com
Internet: http://www.pmeasuring.de