- Automation
Zeta combines stainless steel and single-use
Success for a highly complex automation project
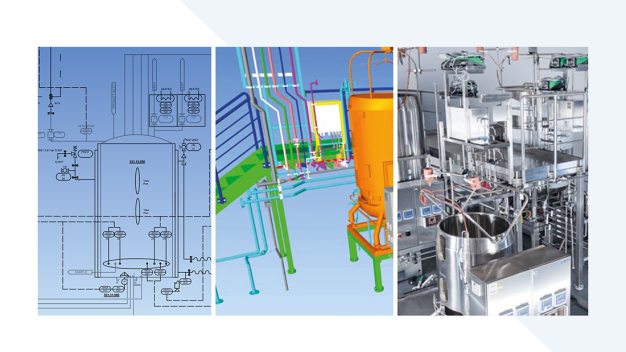
Whether it be stainless steel plant or single-use equipment, as a leading provider of customized automation solutions, ZETA seamlessly integrates biopharmaceutical production systems into new or existing automation environments. A project with a renowned life science company in Germany shows how customer-specific standards are implemented with intelligent automation strategies. For the globally active pharmaceutical company, ZETA automated a stainless steel preparation plant designed and manufactured in-house, as well as a filtration system from a single-use provider, integrating both systems into the overarching process control system.
In the course of the large-scale expansion of an important pharmaceutical production site in Germany – where drug solutions for injection and infusion are manufactured – state-of-the-art technology was applied to ensure the highest production standards. ZETA made its contribution with a customized multipurpose plant consisting of mobile preparation vessels and a cleaning station (CIP/DIP/SIP) made of stainless steel. These were complemented by a filtration system, developed by a single-use supplier, to minimize germ contamination of the product.
The ZETA automation team faced various challenges, as the overall responsibility for the automation of the two flexible systems – stainless steel and singleuse – and their integration with the higher-level process control system lay in their hands.
Automation concept for a smooth process control
The ZETA experts installed the automation technology based on a carefully developed automation concept. For this project, the Siemens PCS7 process control system with SIMATIC Batch was used. The application software designed for the plants was created using the customer-specific programming guideline and the module library specified b y t he c ustomer. D uring t he c onceptual planning stages, special attention was paid to a high degree o f fl exibility o f t he a utomated p rocesses. The finished software system was integrated with the existing customer PCS7 server-client system.
Pharmaceutical production in accordance with GMP and GAMP5
Observing Good Manufacturing Practice (GMP) and complying with standards and regulations is essential in pharmaceutical production. Automation at ZETA follows the Good Automated Manufacturing Practice guideline GAMP5. The creation of batch recipes and batch records – in this project, the SIMATIC Batch software system was used for this purpose – complies with the ISA-S88 standard for batch control. All production plants are centrally monitored and controlled by the process control system. The recipes are created according to the requirements of the respective processes across all plants.
Maximum reliability in the production process
As requested by the customer, automation was designed as a server-client system with a central, redundant control system. The benefit of redundancy lies in the fact that the “spare” system is ready to take over operation in the event of malfunctioning, which means that redundant systems can often prevent downtime. The automation architecture of this extensive project has been designed in a comprehensive way, with the stainless steel and single-use systems programmed by ZETA running on the same controller.
In the completely virtualized process control system, both the visualization and batch servers as well as the centralized programmable logic controllers (PLCs) and the network infrastructure were implemented redundantly. This ensures maximum reliability for the production processes.
Targeted solutions for special automation challenges
Redundancy provides maximum safety during operation. However, it was precisely this requirement for redundancy that called for special automation know-how of the companies involved. Particular attention had to be paid to the system redundancy S2. This specifies the coupling of one or more devices to two redundant controllers and must be guaranteed both physically and in the programming logic. It is essential that the instruments and components that are to be integrated into the bus system are also capable of S2 redundancy, placing the highest demands on the suppliers of the components.
Additionally, during processing, different types of mobile containers must be recognized by this redundant system and docked and undocked from the single-use filtration unit. The undocking process in particular presented a challenge for the ZETA automation t eam. What’s more, the single-use filtration units are mobile, interchangeable with each other and convertible in their mode of operation. The ZETA engineers lived up to this challenge and successfully developed a technical solution for this demanding piece of automation architecture. Part of this is a special coding system in the connectors that transmits power and signals. This way, the respective containers can be identified by means of the digital inputs from the connectors. The result: a user-friendly, safe automation solution for a highly flexible production plant!
A multi-project scheme managed by ZETA
ZETA acted as the overall supplier for the automation of the process. The extensive project was also integrated into the higher-level, highly complex automation architecture at the production site, with the software designed to match. Within the vertical system integration, the ZETA preparation and cleaning system and the single-use filtration system fit seamlessly into the bigger picture, working together with other important system components, such as the ultrapure media supply or the filling system, at the user level.
In addition, t he ZETA automation experts built an interface for the pharmaceutical manufacturer’s Manufacturing Execution System (MES). Such interfaces are particularly important for intralogistics and production control because key economic figures and production data are linked at the MES and Manufacturing Operations Management (MOM) level – the keyword being IT/OT convergence.
Accelerated time-to-market in biopharmaceutical automation
"Time-to-market" is often the top priority in biopharma projects – as a competent solution provider and central contact for automation, ZETA masters this challenge by reducing interfaces, thus achieving shortened project lead times.
A deep understanding of process know-how and the ability to solve even the most complex challenges in automation make ZETA a sought-after partner of the biopharmaceutical industry – this is demonstrated not least by the success of this multi-project scheme which was achieved together with the customer in Germany.
ZETA GmbH
8501 Lieboch/Graz
Austria