- R+D & Community of Interest
Y. Holzapfel and G. Kreck, Fraunhofer Institute for Manufacturing Engineering and Automation IPA, Stuttgart, Germany
Technical cleanliness: Determining particulate cleanliness in fields ranging from the automotive industry to medical device technology
1. Requirements of particulate cleanliness
The need for high levels of particulate cleanliness is obvious for some products such as integrated circuits (with structural widths currently as low as 22 nm) that are manufactured in the semiconductor industry. However, particulate cleanliness, i.e. the absence of micrometer-sized critical particles, is an important quality feature for numerous products in many other industries spanning the automotive industry, through aerospace and right up to life science, such as medical device technology. The reasons for this are as diverse as the products themselves, ranging from improved performance, miniaturization, reliability and durability up to legal requirements and even personal and environmental safety [1].
Although the sizes ranges of critical particles vary from one product to the next and therefore require regulation, some questions overlap, such as:
• How much particulate contamination of which size is on the product?
• What type of contamination is it?
• Can a particle source be identified?
• To what extent can a state of cleanliness be improved by using cleaning technologies?
To answer these questions, different cleanliness analysis methods can be used which are universally applicable.
2. Technical cleanliness: cleanliness analysis in the automotive industry
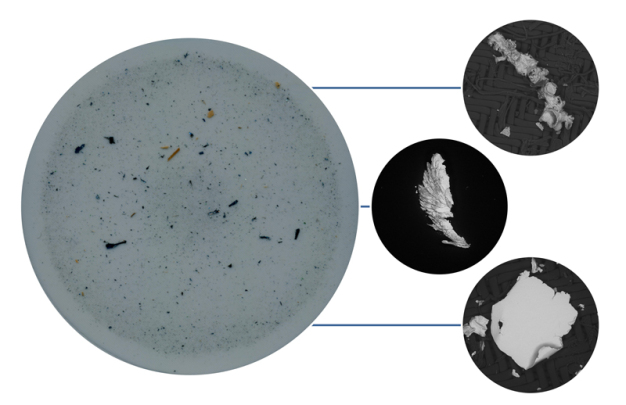
About 15 years ago, subject to the development of more powerful components such as common rail injection systems, the automotive industry started to become affected by particulate contamination. Hard metallic particles cause the most problems (Figure 1).
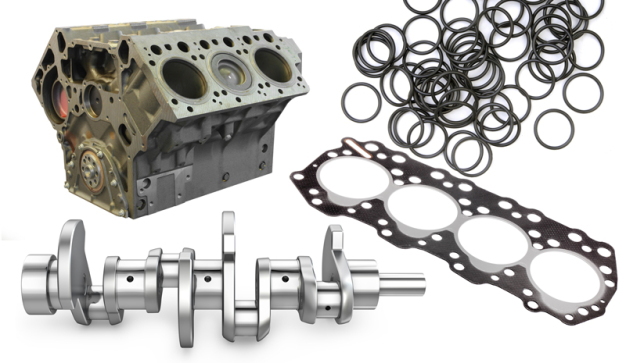
In the automotive industry, the state which describes the absence of functionally-critical contamination on relevant functional surfaces is known as »technical cleanliness«. To ascertain a component’s technical cleanliness, a cleanliness analysis has to be performed. However, due to the range and complexity of automotive components (Figure 2), it is not easy to apply the obvious method of direct inspection to search for potential »killer particles«.
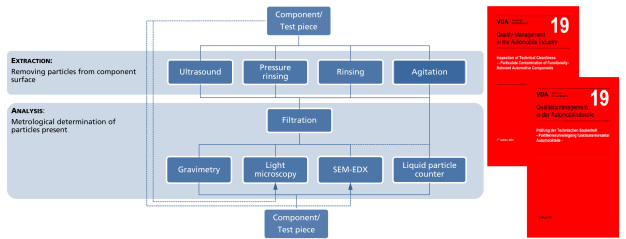
Consequently, a testing method was required that is capable of reliably detecting critical contamination. Under the direction of Fraunhofer IPA, a solution to this problem was jointly developed with participating industrial companies in the industrial alliance »Technical cleanliness (TecSa) «. The fruit of the development was the publication of the guideline »VDA Volume 19 Inspection of Technical Cleanliness - Particulate Contamination of Functionally Relevant Automotive Components«, in which detailed descriptions of procedures and variations of cleanliness analyses for determining particulate cleanliness are given [2]. In this context, a cleanliness analysis can be divided into two steps (Figure 3):
1. Extraction: a liquid process to remove particles from relevant component surfaces with the aid of different techniques (pressure rinsing, ultrasound, rinsing, agitation) (Figure 4) and
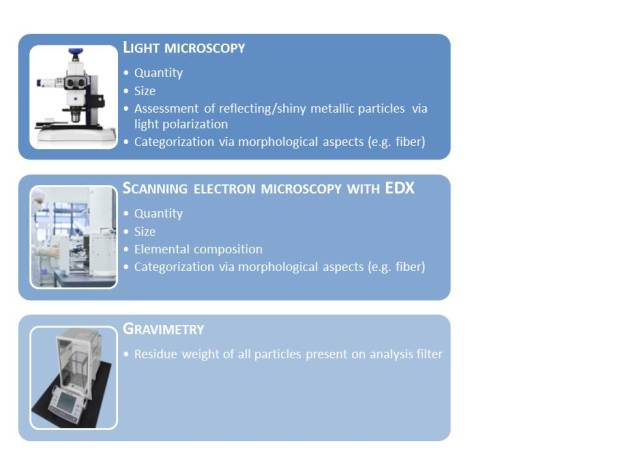
2. The actual analysis, which usually starts with filtration to transfer the particles extracted from the component to an analysis filter. Several different methods can also be used for the analysis, such as gravimetry (determination of residue weight) and automated microscopic techniques (light microscopy or scanning electron microscopy combined with energy dispersive x-ray spectroscopy EDX, Figure 5).
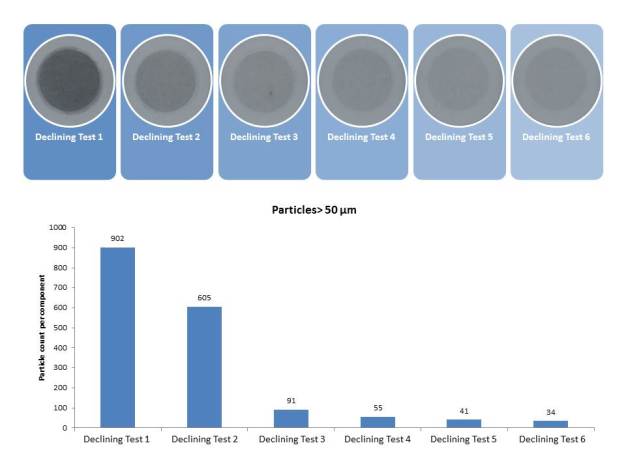
As well as the blank value, which also has to be ascertained for numerous other analytical determinations, it is especially important to assess the suitability of the extraction parameters chosen. Because no »normed contaminated components« (= components with a known uniform initial state of contamination) exist which would allow cleanliness analysis results with different parameters to be compared with one another, a so-called qualification test or declining test is carried out. Here, the same component is repeatedly subjected to an extraction process. The aim is to remove 90 % of all particles present within a maximum of six successive extraction steps (Figure 6):
• If this is already the case after the first declining tests, the chosen parameters can be kept.
• If values only decline after further extraction steps, parameters are adjusted for the routine inspections to be carried out later on, e.g. by lengthening the duration of the extraction process.
• If no declining values are obtained, another declining test is performed using more appropriate parameters or a different technique.
3. Problem of recontamination due to assembly
The observation that technically clean single components do not guarantee a clean overall system underlines the need to ascertain which factors influence product cleanliness in assembly.
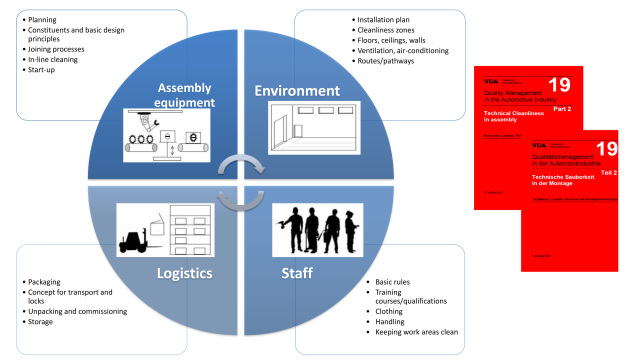
These factors were determined in the industrial alliance »MontSa« and documented in the guideline »VDA Volume 19.2 Technical Cleanliness in Assembly - Environment, Logistics, Staff and Assembly Equipment« [3]. The guideline describes influencing factors, such as the environment, staff, logistics and assembly equipment, from the point of view of cleanliness in assembly with the aim of identifying potentials for optimizing cleanliness in a production facility, or of designing it in a cleanliness-orientated way right from the beginning (Figure 7).
The main basic principles are:
• »from inside to the outside«, i.e. when taking optimization measures, the initial focus should be on processes taking place close to the product, and
• »as clean as necessary, not as clean as possible«, i.e. transferring production to a cleanroom often does not achieve the desired result of clean products because the sometimes millimeter-sized assembly chippings are too heavy to be airborne and therefore cannot be removed by the airflow in the cleanroom.
Consequently, an important starting point for optimizations is the identification of particle sources in the assembly environment. This is achieved using methods such as Tape Lift to ascertain the cleanliness of surfaces, or particle traps to capture particles sedimenting on an adhesive pad in order to monitor the environment in various areas of the production facility. Particle traps can also be utilized to ascertain the contamination potential of processes (Figure 8).
The same analysis techniques are used as for analyzing component cleanliness (automated microscopic analysis with light and scanning electron microscopy). In certain cases, by identifying particle sources it may be possible to avoid contamination (e.g. by designing assembly equipment appropriately) or to remove it effectively (e.g. by integrating a cleaning process into assembly).
In order to minimize particle generation and contamination transfer, a further focus is placed on supplementary suitable logistic concepts, such as cleanliness-orientated packaging or suitable lock concepts.
Staff members are also considered because they can decisively influence the state of cleanliness of a product. The workers concerned may be responsible for generating the contamination or for removing it.
4. Applying methods to life science products
Despite the fact that the cleanliness issue is deeply rooted in the life science sector, especially in pharmaceutics, and that national and international standards and legal requirements have been in existence for some years now, problems due to insufficiently clean products still occur.
Medical technology products are one example of this. Over the last ten years, approx. 250 medical technology products have been recalled by the Food and Drug Administration (FDA), with approx. 30 % of these being due to contamination [4].
One explanation for this is the currently inadequate verification of the level of particulate cleanliness. At the moment, the main emphasis of cleanliness analyses performed on these products is on assuring sterility, which is often wrongly equated with the absence of particles. Particulate contamination entering the human body is also associated with a risk potential because it may have a toxic or pyrogenic effect. A logical consequence would be to continuously monitor and reliably verify the state of particulate cleanliness. However, at the moment there are a number of deficits in this respect. One of them is limiting values for particulate contamination; these were originally conceived for injection and infusion fluids but have since been transferred in part to a whole range of medical products. Another deficit concerns determining cleanliness levels because specific test methods exist only for a handful of medical technology products.
To determine particulate contamination, various norms e.g. [5], [6], [7], [8] describe the following procedures:
1. Testing by means of liquid particle counters
2. Filtration and manual analysis with a microscope
A critical aspect here is the extraction step: in order to collect contamination from a product, a rinsing method is described for infusion devices, for example, and a swilling method using a test fluid for elastomer components in parenterals [6]. These product-specific methods cannot be generally applied to the broad spectrum of medical products (Figure 9) without first assessing their suitability. Only after procedures have been validated can their application be appropriately modified for other products. Methods can be validated by means of a declining test, such as that described in VDA 19 for inspecting automotive components.
To support the identification of contamination sources, automated systems as well as analysis techniques could be utilized to characterize particles in more detail (e.g. SEM-EDX to determine elementary composition).
5. Cleaning processes as a control strategy
Cleaning steps are often necessary to control contamination. Depending on the product and manufacturing process concerned, it may make sense to carry out a cleaning step at various stages of the production process, e.g.
• At individual component level because they should/must be clean for a specific reason,
• Integrated into assembly in order to directly remove contamination generated by assembly processes or
• At the end of production when the system is complete.
The efficacy of the cleaning technique used can be verified by means of a cleanliness analysis.
In cases where a comparison between different cleaning methods is required, an assessment matrix can be used which takes, among others, the following points into account:
• Investment and operating costs
• Compatibility with the product to be cleaned
• Environmental aspects
• Cleaning efficacy
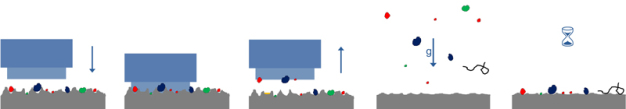
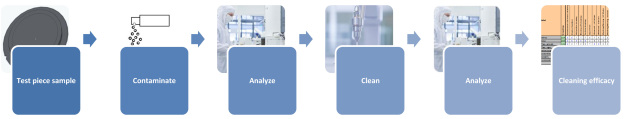
Especially when ascertaining the efficacy of different cleaning methods, it makes sense to work with test pieces that allow contamination to be determined directly without extraction losses. After contaminating test pieces with a tracer in a defined way and then analyzing them, they are cleaned using one of the various techniques. Cleaning efficacy is calculated quantitatively by carrying out a second analysis of the test piece after cleaning to ascertain the residual amount of tracer present (Figure 11).
6. Summary and further activities
The life science industry, in particular the sectors of pharmaceutics and medical technology, is required to validate all its procedures. Especially in the area of particulate cleanliness analysis, there is often a lack of suitable detection procedures, with the consequence that strongly-deviating non-comparable results tend to be obtained.
It was possible to demonstrate the application of the VDA 19 procedure to cleanliness-sensitive medical technology products in several tests [10], [1]. To discuss these aspects as well as other cleanliness-related problems in a dialog, Fraunhofer IPA is now inviting people to participate in an event with a podium discussion that is scheduled on 25th February 2014 in order to identify – together with industry - the focus of future research and standardization work (http://www.ipa.fraunhofer.de/Braucht_die_Medizintechnik_neue_Ansaetze_fuer_die_Reinheitsvalidierung.2581.0.html). Also a survey on the topic of validating cleanliness in medical technology: www.ipa.fraunhofer.de/medizintechnik.
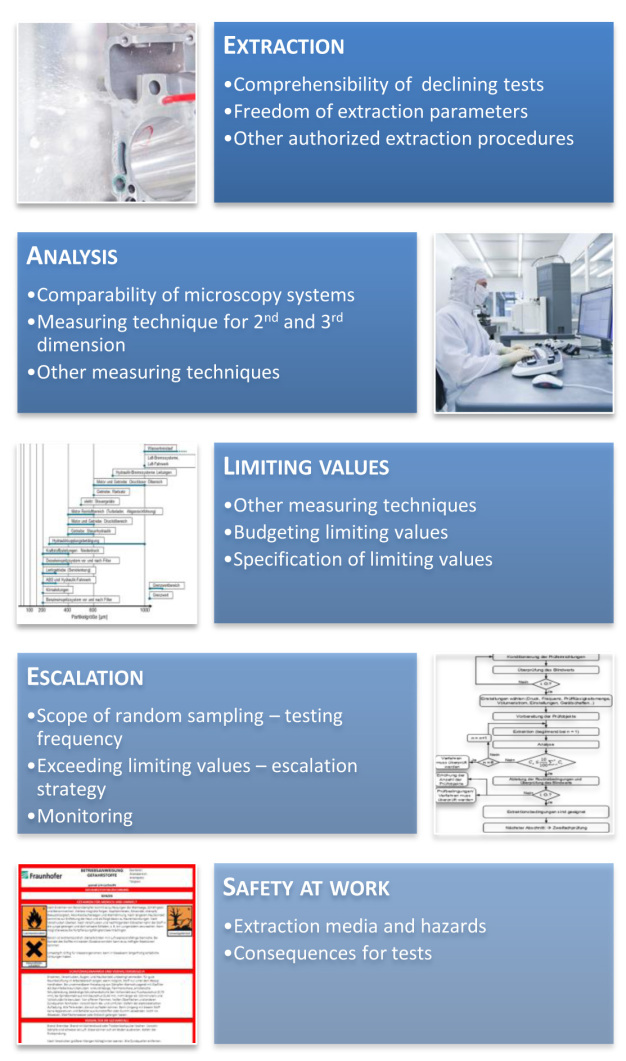
This approach has already proved its effectiveness in the automotive industry: through continuous discussions with relevant partners, VDA 19 is currently being revised subject to the formation of an industrial alliance with 40 industrial member companies. This enables the needs and requirements of industry to be mapped according to the state of the art, taking the knowledge gained from the VDA 19 project into account. The book is being revised by several different work groups divided into subject groups (extraction, analysis, limiting values, escalation, Figure 12).
7. Training courses
A further very important point is the motivation of workers carrying out cleanliness-related tasks. Targeted training courses can make employees more aware of the various issues related to cleanliness. In collaboration with VDA QMC, the following training courses are available:
7.1 Training course to become a »Technical Cleanliness Inspector« (VDA 19)
Topics:
The technical cleanliness of components and aggregates is an important functional quality feature in the manufacture of modern vehicles. The first comprehensive standardization work of its kind, the book »VDA Volume 19 Inspection of Technical Cleanliness - Particulate Contamination of Functionally Relevant Automotive Components« addresses methods and procedures for characterizing the cleanliness of products in the automotive quality chain.
Aim of the course:
In a unique training course given by Fraunhofer IPA in collaboration with VDA QMC, people are trained to carry out technical cleanliness inspections. Participants learn not only to independently configure cleanliness analyses in accordance with VDA 19 but also to carry them out with the aid of state-of-the-art equipment and document results compliant with the relevant regulations.
Target group:
The course is aimed at people who are involved with construction, quality assurance, technical purchasing and sales in the automotive and supplier industries, in aerospace, hydraulics and precision engineering, or are responsible for carrying out cleanliness inspections or confronted with the quality characteristic of »technical cleanliness«.
Next course (course language German):
4th to 5th February 2014
7.2 Training course to become a »Technical Cleanliness Planner« (VDA 19.2)
Aim of the course:
The course teaches participants how to derive and assess measures to prevent re-contamination that are based on component or system cleanliness specifications. The structure of the guideline and the training program allows the comprehensive cleanliness planning or optimization process to be divided into separate compact, manageable packages. By dealing with the influencing factors of environment, logistics, staff and assembly equipment as well as individual and general methods for measuring cleanliness influences, participants learn how to approach technical cleanliness in assembly in an independent and systematic way. This helps them recognize inappropriate or excessive cleanliness measures and avoid misinvestments.
Target group:
The course qualifies people working in the automotive and supplier industry to plan and optimize production with regard to technical cleanliness. It is especially aimed at assembly planners as well as process owners of existing assembly and logistics technologies and building services. The course is also intended for design engineers and developers, quality controllers and people responsible for technical cleanliness in customer-supplier relationships. Because they are confronted with similar cleanliness issues, it is also suitable for people active in the fields of aerospace, hydraulics and precision engineering.
Next course (course language German):
11th to 12th March 2014
Bibliography
[1] L. Gail, U. Gommel, H.-P. Hortig: Reinraumtechnik, 3rd Edition, Springer, Berlin, Heidelberg, 2012.
[2] Verband der Automobilindustrie e. V. (VDA): Volume 19 Inspection of Technical Cleanliness – Particulate Contamination of Functionally Relevant Automotive Components, 1st Edition, 2004.
[3] Verband der Automobilindustrie e. V. (VDA): Volume 19.2 Technical Cleanliness in Assembly – Environment, Logistics, Staff and Assembly Equipment, 1st Edition, 2010.
[4] U.S. Food and Drug Administration: Recall of Medical Devices. URL: http://www.fda.gov/MedicalDevices/Safety/ListofRecalls/default.htm, invoked on 27th June 2013.
[5] European Pharmacopoeia (Ph. Eur.): Methoden der pharmazeutischen Technologie, Kapitel 2.9.19: Nicht sichtbare Partikel, 7th Edition, 4th Amendment, 2013.
[6] ISO 8536-4: Infusion equipment for medical use - Part 4: Infusion sets for single use, gravity feed, 2013.
[7] ISO 1135-4: Infusion equipment for medical use - Part 4: Infusion sets for single use, 2012.
[8] ISO 16671: Ophthalmic implants – irrigating solutions for ophthalmic surgery, 2004.
[9] DIN EN ISO 8871-3: Elastomeric parts for parenterals and for devices for pharmaceutical use – Part 3: Determination of released-particle count, 2004.
[10] EUMINAfab: Your gateway to micro nano fabrication. URL: http://www.euminafab.eu/, invoked on 27th June 2013.
[11] G. Kreck und Y. Holzapfel: Sauberkeitsanalyse und Präzisionsreinigung von reinheitskritischen Life Science Produkten. In:15. VDI-Fachtagung "Reinraumtechnik", 13th June 2013.
![]()
Fraunhofer-Institut für Produktionstechnik und Automatisierung IPA
Nobelstraße 12
70569 Stuttgart
Germany
Phone: +49 711 970 1667
email: joerg-dieter.walz@ipa.fraunhofer.de
Internet: http://www.ipa.fraunhofer.de