Nephron Pharmaceutical Compounding Facility Integrates STERIS VHP Biodecontamination System
A Case Study Examining the Benefits and Challenges of Integrating VHP® Biodecontamination into a High-Volume Compounding Facility
Background
Nephron is a rapidly growing manufacturer of generic respiratory medications and a contract manufacturer of blow-fill-seal products. Also, as a 503B compounder, Nephron has made significant investments in the production of high-volume pharmaceutical preparations for interstate distribution. Adapting their production output to the constantly changing needs of the market requires frequent product change over activities. Pharmaceutical compounding is a time and labor-intensive venture. Production processes are strictly regulated for quality and safety. Workers require a high level of training and to meet demand, facilities remain in continuous operation.
To meet the aseptic standards associated with this type of work, Nephron spends a considerable amount of time and resources cleaning and disinfecting large production spaces. In 2018 they embarked on a joint project with STERIS to investigate the use of an integrated vaporized hydrogen peroxide (VHP) biodecontamination system to reduce the time associated with manual disinfection. This was done with the intent of streamlining their validated, manual “triple clean” sanitization process. The triple clean process involves several hours of manual cleaning with formulated chemistries to clean and sanitize large cleanroom spaces. Since initiating the investigation of VHP, Nephron has also undertaken a major expansion of its production capacity.
This case study will provide insight into Nephron’s experience adopting an integrated VHP approach, the challenges encountered, and results achieved.
Nephron’s Existing Decontamination Process
Nephron performs several different sanitization steps daily. These include start-up cleaning, routine medial cleaning, scheduled cleaning, gross and triple cleaning. With multiple lot changeovers per day – the required sanitizations must be performed in several different rooms simultaneously sometimes resulting in scheduling conflicts. During the triple clean process, Sterile Room Technicians (SRTs) must utilize three different cleaning agents on all surfaces within the space, while waiting the appropriate contact time between each application.
Nephron’s Initial Contact with STERIS
In the Summer of 2019, Nephron’s CEO, Lou Kennedy, learned of the STERIS integrated VHP biodecontamination system. Integrated VHP was discussed and endorsed within Nephron’s management team and regarded as a technology worthy of further investigation. Sometime thereafter, Kennedy announced the potential collaboration with STERIS at the ISPE meeting in Las Vegas, Nevada. In her presentation, she put forward her belief that integrated VHP technology would significantly contribute to Nephron’s goals of developing the “facility of the future”.
Nephron’s Sanitization Requirements
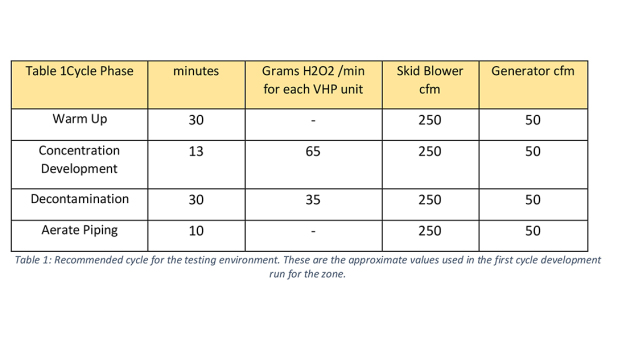
Initial discussions between teams from each company determined that Nephron needed an automated biodecontamination solution for their manual and automated filling suites. Nephron has six distinct zones that require frequent decontamination. Nephron’s maximum target cycle time, for the entire VHP biodecontamination cycle (including the aeration phase), was 6-hours.
Introduction to VHP - Process Evaluation
STERIS and Nephron began feasibility testing with two STERIS portable Biodecontamination Units. Feasibility testing introduced the Nephron team to the process and benefits of working with a gaseous decontamination agent, providing them with valuable insights to develop a detailed user requirement specification (URS).
Nephron Internal Evaluation and Decision
Nephron trialed other decontamination technologies. STERIS VHP was selected because of its ability to decontaminate large spaces and, perhaps more importantly, its ability to integrate with their Building Automation System. In a 503B manufacturing facility such as Nephron’s,most cleaning operations are manual and it can be challenging to source, train and retain staff to do the job correctly. In contrast, the ability automate decontamination can reduce operating costs, improve flexibility, and ensure process consistency.
Nephron also chose to work with STERIS because of the company’s technical expertise and integration support services. These services include, cycle development assistance, operator training, installation support, factory acceptance testing and site acceptance testing, installation qualification/operational qualification, preventative maintenance, and calibration. No other company offered the same track record of success or level of technical expertise. STERIS sent an integrated application proposal to Nephron in September 2019. This included a basic piping layout with proposed airflows and cycle times. Nephron placed an order for two high-powered VHP units in December of 2019. Nephron’s goal was to receive the units in March 2020 for installation in early April 2020.
Application Feasibility Assessment
Nephron wanted a unit that could decontaminate any suite in their facility with the shortest possible cycle time. This would require a system with very short warm up times and the ability to deliver near peak injection rates through hundreds of feet of piping without condensation. Therefore, the STERIS VHP generators were run in tandem in the same space to further shorten cycle time. Due to the length of the piping, pressure loss, concentration and temperature calculations were made to assure that the VHP could reach the target zone as a vapor and be evenly dispersed. Modifications were also made to the overall implementation to lessen the effect of warming during the VHP cycle.
Manufacturing at STERIS
Once the technical details were finalized on-site at Nephron, STERIS engineers could begin the manufacturing process of the two VHP integrated generators and the accompanying accessory skid. The manufacturing process began in late January 2020 and by February the units were ready to go through a series of vigorous final testing requirements. After passing a series of performance tests, the units shipped in March 2020.
The Integration Plan
As with any gaseous decontamination process involving diverse enclosures and system components, there are bound to be uncertainties. In this case, STERIS had yet to transport this volume of vapor through this distance of CPVC piping without heat tracing. The use of heat tracing hundreds of feet of piping would have added significantly to the cost. It was also uncertain whether, post decontamination, the air handling units could be efficiently adapted to aerate the peroxide quickly enough to achieve the 6-hour cycle time. Nephron’s strategy to address these uncertainties was to dedicate one of their suites, in their facility, for testing and developing standards for future VHP integration. To that end, this “testing environment” would be modified and run with multiple VHP cycles to create a process for the modification of other suites in their facility.
Nephron invested approximately $150,000 to modify their facility to support this automated decontamination process. They installed valves and VHP injection ports, enhanced the volume of makeup and exhaust air in the plenum of their testing environment and added damper controls to supply and return air. They also added maglocks to doors, an emergency exit button for safety. Rooms and plenum space were smoke tested, and any leaks were sealed. The VHP units were then installed in Nephron’s mechanical space and a contractor installed the piping and manifolds to distribute the VHP throughout the facility.
IQOQ
After installation, trained STERIS team members tested the VHP systems’ onboard hardware and software to ensure the quality and functionality of the product. IQOQ documentation was developed that exceeded the FDA’s requirements to help facilitate future regulatory audits.
The IQOQ had to be performed on both systems, which were installed in Nephron’s mechanical space. The temperature in this space, is typically around 86°F and STERIS engineers made certain the equipment would function at such high temperatures. Throughout testing and the IQOQ process, the systems functioned without issue.
The Initial Test Cycle
Prior to any additional facility modifications, a VHP test run was completed in the dedicated testing suite to determine how the system performed with the room in its current state. To begin, a cycle was run with distilled water. This was done to determine temperature increase in the distribution piping and confirm that differential pressure to surrounding areas could be
maintained through each cycle phase transition.
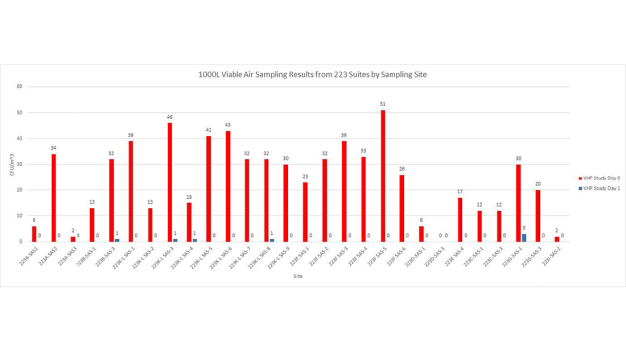
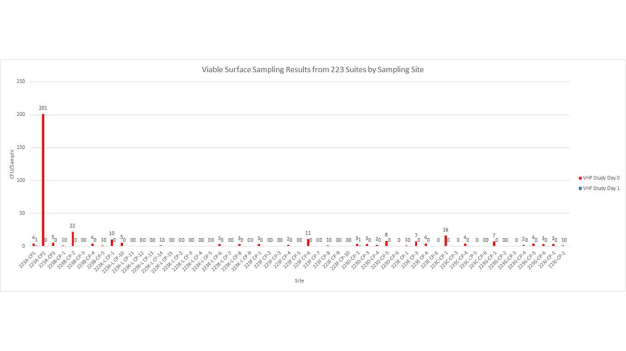
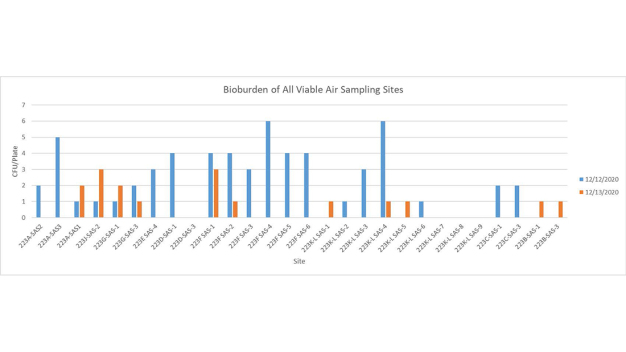
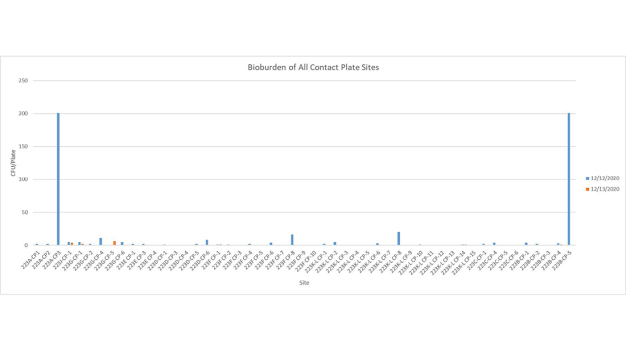
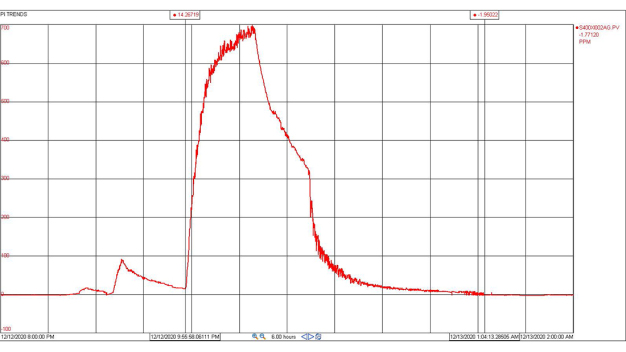
The first cycle with VHP had to be aborted mid-way to make some necessary system adjustments. The fan filter units were left running during the cycle which caused a slow increase in peroxide concentration levels. It seems likely VHP was being inadvertently exhausted from the plenum. While this test was unsuccessful, it did generate initial environmental data that was very encouraging (Figures 6 and 7).
The system was adjusted to prevent the fans from remaining on during the decon cycle and, prior to the next test, Nephron made modifications to their HVAC system to optimize the decontamination cycle. These modifications included higher single pass air flows for improved aeration and better control over the fan filter units and the cooling coil. An additional valve was added to the VHP distribution manifold to isolate the generators and provide system redundancy if one of the units were to abort during the cycle.
First Completed Cycle
With the knowledge gained during the previous test and subsequent modifications to the system, a successful VHP decontamination cycle was completed on the next attempt. Moreover, the test cycle was completed in 3 hours and 2 minutes, nearly 50% faster than the original targeted cycle time of less than 6 hours. Further the data indicated that the environmental monitoring targets had been reached (Figures 8 and 9).
Although the results are preliminary, the project team feels confident that a 3-hour cycle time is achievable. Reasons for this dramatic reduction in cycle time include:
1. Two generators injecting into the suite at once warms the line and builds concentration quickly.
2. Injecting directly into the rooms without recirculating with the fan filter units during the injection phase also helped to quickly build concentration.
3. Integrating the VHP units into the building automation system, operators were able to monitor the cycle and increase the injection rate when concentration levels were dipping.
Realized Benefits of VHP Integration
The results, of the first phase of integration, are extremely promising. Going forward, the STERIS system will be a valuable tool to significantly reduce manual cleaning time and to improve efficacy due to the automated nature of VHP decontamination. In addition to the automation benefits, the target cycle time of 6-hours was cut in half. Data generated to during testing, supports the premise that short exposures of high concentrations of VHP can effectively reduce both air borne and surface counts of viable microorganisms. This means the time-consuming manual cleaning process could now be completed in less than 3-hours. STERIS and Nephron will continue to work together to implement this “facility of the future” biodecontamination system into the rest of their process.
STERIS Deutschland GmbH
50933 Köln
Germany