Selecting the right hand protection when working with chemotherapy drugs
Manufacturing specialized products in a pharmaceutical manufacturing environment has a specific set of requirements when it comes to hand protection. Production and handling of these types of products typically happens in a clean or controlled environment and quite often in a sterile environment as well. When this particular application is further specialized into manufacturing or handling of a particular set of pharmaceuticals known as chemotherapeutic agents additional requirements come into play when selecting appropriate hand protection.
This paper will explore these requirements and what an individual who is working in these environments should be concerned with, while selecting appropriate hand protection as part of their overall personal protective equipment ensemble. It should be noted that this paper discusses the use of gloves as personal protective equipment in an industrial or non-medical application. For those individuals who are handling chemotherapeutic agents in a medical setting additional regulatory requirements must be met.
There are two primary reasons to wear personal protective gloves when working with these types of drugs. First and foremost to protect the individual from exposure to a potentially harmful substance and secondarily to protect the product from contamination. Chemotherapeutic agents are a class of chemical compounds designed and formulated as a drug product to inhibit the growth of or destroy rapidly growing cancer cells within the body. Therefore, by definition, they are either cytostatic or cytotoxic compounds and as such require the use of personal protective gloves that will act as an effective barrier between the hand and the chemical compound in question. Since these compounds are by nature destructive to human cells it is desirable to avoid exposure to these compounds.
Determining whether a glove provides adequate protection
How then does an individual working in these environments and potentially exposed to these types of chemical compounds know whether or not the gloves they are wearing will provide adequate protection? Gloves designed to be used in these environments can be evaluated for their protective qualities when in contact with chemical substances. This is done by conducting what’s known as a chemical permeation test and is conducted under the guidance of two US industry consensus standards. These standards are known as ASTM D6978 Assessment of Resistance of Medical Gloves to Permeation by Chemotherapy Drugs and ASTM F739 Standard Test Method for Permeation of Liquids and Gases through Protective Clothing Materials under Conditions of Continuous Contact respectively. Whereas ASTM F739 is the general test method used to conduct chemical permeation testing, ASTM D6978 includes some additional requirements specific to chemotherapy drugs.
Standard test methods
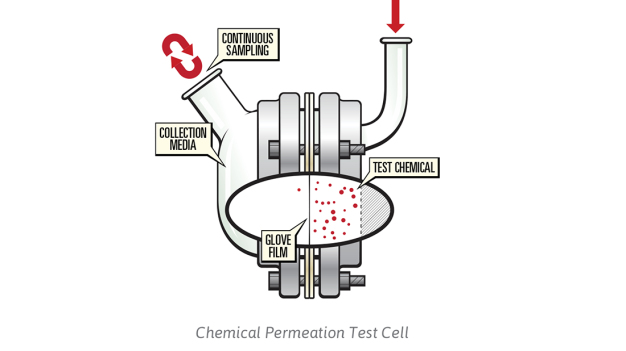
The ASTM F739 standard test method is used to identify the actual chemical permeation resistance of glove materials under continuous contact with chemicals. The glove material to be tested is placed into a permeation test cell and sandwiched between the test chemical and a collection medium. The collection medium, usually a gas or liquid, is analyzed quantitatively for its concentration of the chemical that has permeated the barrier as a function of time after its initial contact with the glove material.
Each material specimen to be tested is sampled from the palm of at least three gloves. An additional sample may be tested with just collection media as a test control depending upon the actual analytical methods used. All test specimens are cut to fit the same diameter as the flange of the permeation test cell (see Figure 1).
The test chemical is introduced into the challenge compartment of the permeation cell and the time measuring device is started. The compartment containing the test chemical is completely filled during the period of the test. Under the requirements of ASTM F739 the breakthrough time of a chemical is deemed to occur when the sum of the permeation rates of each individual component reaches the rate of 0.1μg/cm2/min. When a permeation rate of 0.1μg/cm2/min is detected, then the breakthrough time is reported in minutes for each test specimen. If the permeation rate does not reach 0.1μg/cm2/min then the duration of the test is reported.
However, for chemotherapy agents under the additional requirements of the standard ASTM D6978 a more conservative breakthrough time is reported by determining a breakthrough time when 0.01μg/cm2/min is reached. This is done in recognition of the cytotoxic/cytostatic properties of the chemical compounds in question.
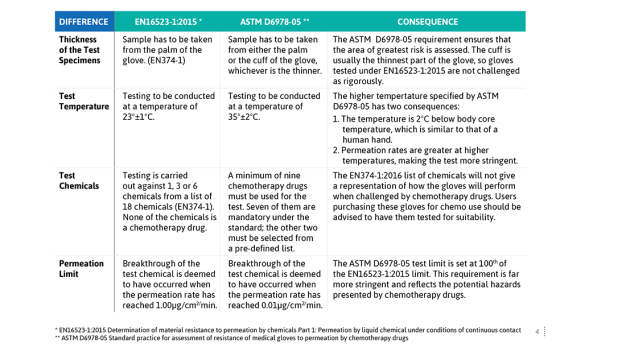
Why Ansell does not use the test method EN 16523-1:2015 as set out in the EN ISO 374 standard when testing against chemotherapy drugs
Ansell gloves are tested against the most stringent standard, the American ASTM D6978-05 which employs a testing limit 100 times more stringent than its European counterpart. We do not test gloves using the EN16523-1:2015 (formerly EN374-3) method as this benchmark is not safe when assessing the suitability of a glove for protection against chemotherapy drugs.
To illustrate how the two standards parameters compare Ansell has highlighted the consequences in table 1.
Product contamination concerns
While personal protection is the first concern when selecting a glove, protecting the product from external sources of contamination is equally important. Manufacturing of chemotherapy drugs is conducted under good manufacturing practices (GMP) in a sterile cleanroom environment and as such, product contamination must be avoided. A variety of sources of potential contamination must be taken into consideration, including biological, particulate and undesirable chemical residues. A contaminated product from any of these sources can lead to unacceptable production lots resulting in a costly and time consuming scenario to rectify.
Recommended solutions
How is an appropriate glove chosen for use with chemotherapy agents? As this paper illustrates several factors need to be taken into consideration.
- Protection against:
» specific drugs being used
» other hazards or chemicals in the work place
- Protection of the products from external contamination
- Comfort
- Fit
- Ergonomics
- Costs
Additionally, a common practice of wearing two pairs of single use gloves (double donning) can also enhance the end user’s protection against chemotherapy agents provided the gloves are chemotherapy drug approved and proven to be elastic and comfortable. In consideration of all these factors Ansell has several product offerings that fulfill these challenging and very specific needs of this environment.
Glove box environment solutions
Glove boxes play a vital role in protecting products from human or environmental contamination as well as protecting individuals and environments from hazardous chemicals used for the compounding of chemotherapy drugs. Due to the propensity of sensitive materials utilized in the life sciences, any of three different types of glove boxes may be used; Containment glove boxes, Isolation glove boxes and Isolators. The environment inside a glove box is typically sterile, clean and pressurized, either positively or negatively, to meet the specific requirements of the application.
Isolators are used to contain some of the most dangerous and toxic material known to man, therefore they are ultra-clean and contained for product and personal protection.
Ansell Life Sciences isolator glove solutions have been tested to the most stringent standard ASTM D6978 against chemotherapy drugs, to ensure the greatest possible protection.
Permeation: is the process by which a chemical dissolves and/or moves through a protective glove material on a molecular level. Permeation can occur without damaging the material or by damaging the material by degrading it. Permeation is measured in the amount of time (minutes) it takes for a chemical to pass through the barrier at a determined permeation rate, which is referred to as Chemical Breakthrough Time; and the Permeation Rate which is the rate (volume over time) at which a chemical passes through the glove material.
Penetration (break-through): is the movement of a chemical and/or microorganism through the material, pinholes or other imperfections of a glove.
Degradation: is the loss of, or change, in the glove material’s chemical resistance or physical properties due to exposure to chemicals and/or use. These changes can occur as swelling, disintegration, becoming brittle, discoloration, flaking, hardening, or softening and is measured by taking before and after results of different metrics such as tensile strength, force at break, modulus, visual observation, and other metrics.
Ansell GmbH
81829 München
Germany