Wann ist ein Reinraum Handschuh ein "Reinraum-Handschuh"?
Die Validierung eines Handschuhes, der im Reinraum eingesetzt werden soll, ist ein langer und anspruchsvoller Prozess. In Anbetracht der Tatsache, dass die Reinräume immer strengeren Regeln unterliegen, hatte dies auch dramatischen Einfluss auf die Anforderungen an Einweghandschuhe. Vor 20 Jahren war es noch normal, Standard Handschuhe einfach in PE Beutel zu verpacken und dann als Reinraumhandschuhe einzusetzen. Es waren vorzugsweise Vinylhandschuhe im Einsatz, speziell in der Halbleiter-Industrie. Ein immer besser werdendes Verständnis von Kontamination und Barrierenfunktion haben dazu geführt, dass diese Handschuhe mehr und mehr vom Markt verschwunden sind. Parallel dazu ging die Entwicklung in der Pharmaindustrie vom Standard Operationshandschuhe in Papierverpackung zu Operations-Handschuhen in PE Verpackung – gerade für den Einsatz im aseptischen Bereich.
Nach Einführung des ISO 14644-1 Anfang 2000 gab es endlich einen internationalen Standard für die Reinraum Klassifizierung und machte das Verständnis für Partikelreinheit in der Luft um vieles einfacher. Eine ähnliche Annäherung fand in Europa in der Pharmaindustrie statt durch die Publikation des “Guide to Good Manufacturing Practice for Medicinal Products and its Annexes“ (EC GMP). Speziell die Hersteller von sterilen Medikamenten, Infusionen und Impfstoffen usw. folgen den Anhängen des GMP für medizinische Produkte. Während keine dieser Entwicklungen den Fokus auf die vielen Einwegprodukte legte, die im Reinraum verwendet werden, ging der Trend immer mehr dazu, reinere Produkte zu suchen und einzusetzen – eine klare Anforderung einer reineren Umgebung an die Verbrauchsmaterialien. Als möglichen Weg aus dieser Vorgabenlücke hat sich der VDI darum bemüht, dass zumindest Basisanforderungen in VDI Reinraum Technologie Verbrauchsmaterialien 2083 Blatt 5.1 Anhang H abgedeckt werden.
Quer durch Elektronik-, Luftfahrt- und Solarindustrie wurden die Evaluierungsgrundlagen für Einwegprodukte im Reinraum gravierend verändert und die Anforderungen verschärft. Leider fanden diese Veränderungen nicht mit der gleichen Geschwindigkeit in der Pharmaindustrie statt. Hier werden immer noch in einigen Bereichen einfach sterile Operationshandschuhe (aseptische Bereiche) und Standard Untersuchungshandschuhe (in nicht sterilen Bereichen) eingesetzt. Viele Krankenhausapotheken, in denen Zytostatika Substanzen, Infusionen, Parenteralen hergestellt werden, arbeiten aus Kostengründen noch immer mit Klinikhandschuhen. Es wird oft immer noch zu wenig auf Sicherheit der Mitarbeiter, des Produktes und des Umfeldes geachtet. Durch den Einfluss der Vorschriften von ISO14644 und speziell der Vorschriften der EC GMP fangen auch diese Bereiche an, sich mit den notwendigen Veränderungen auseinander zu setzen.
Warum brauchen Reinräume einen speziellen Reinraumhandschuh?
In vielen Fällen wird das Tragen von Handschuhen vorgeschrieben um das Produkt vor Kontamination zu schützen. Neben den hohen Kosten für Produktionsausfälle durch Kontamination kann die Kontamination mit biologischen Erregern bei Parenteralen, Infusionen usw, schwere Konsequenzen für die Patienten haben. Eine ähnliche Gefahr geht vom Umfeld im Reinraum aus – auch eine Verunreinigung im Umfeld kann garvierende Folgen für das Produkt und damit den Patienten haben.
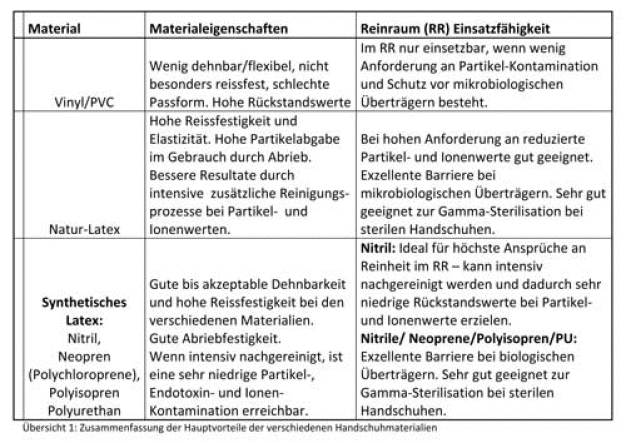
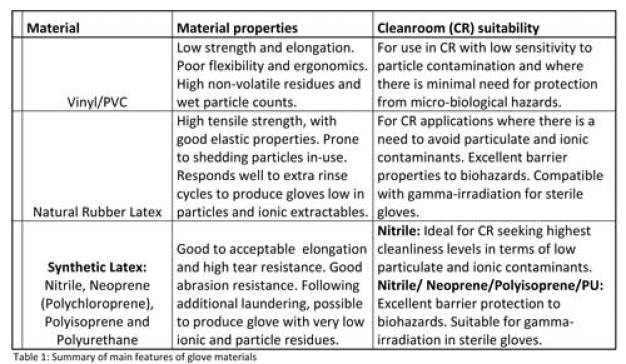
Der erste Schritt der Evaluierung ist die Auswahl der Handschuhmaterials
Siehe Übersicht 1: Zusammenfassung der Hauptvorteile der verschiedenen Handschuhmaterialien.
Bereiche, in denen ESD eine wichtige Rolle spielt, sollten folgende Punkte beachten:
- Natur Latex Handschuhe sind statisch insulativ – statisch insulative Materialien haben einen sehr hohen Oberflächenwiderstand – höher als 1 x 10¹² ohms/square. Die grosse Gefahr ist hier, dass sich das Material enorm auflädt und diese Ladung dann unkontrolliert wieder abgibt. Selbst wenn die Oberfläche eine hohe Ionen-Kontamination aufweisst, verhält sich der Handschuh statisch insulativ.
- Nitril Handschuhe haben einen Oberflächenwiderstand, der sich zwischen statisch insulativ und statisch dissipativ bewegt. Intensive Nachreinigung mit DI Wasser bringt den Oberflächenwiderstand mehr in die Region des statisch dissipativen Verhaltens. Statisch dissipativ bedeutet hier, dass eine vorhandene Ladung auf kontrolliertem Wege abgeleitet werden kann und nicht zu einer Zerstörung des Produktes führt. Als statisch dissipative bezeichnet man einen Handschuh dann, wenn sich der Oberflächenwiderstand im Bereich 1 x 10⁵ aber niedriger als 1 x 10¹¹ ohms/square bewegt.
- Vinyl Handschuhe (in der Vergangenheit auch oft als ESD Handschuhe bezeichnet) bieten die besten Eigenschaften beim Oberflächenwiderstand – offensichtlich durch die sehr hohe Oberflächenkontamination mit Ionenrückständen.
- Neopren/Polyisoprene-Handschuhe haben möglicherweise die gleichen ESD Eigenschaften wie Nitril. Problematisch ist zu sehen, dass die meisten Neoprenhandschuhe eine Innenbeschichtung aufweisen (PU oder Silicon). Diese Innenbeschichtung erleichtert das Anziehen auf angenehme Weise, haben aber den Nachteil, dass eine Nachreinigung mit DI Wasser nicht möglich ist. Dies führt zu stärkerer Rückstandsbelastung auf der Oberfläche. Es ist anzunehmen, dass das ein eventuell akzeptables ESD Verhalten möglicherweise durch die Oberflächenbelastung kommt.
Warum ist es wichtig, auf die Reinraum Klassifizierung zu achten?
Gemäß ISO 14644-1, ist es so, dass je niedriger die ISO Klasse ist, umso weniger luftgetragene Partikel sind erlaubt. Während sich die Elektronik Industrie auf Partikel und auslösbare Rückstände konzentriert, konzentriert sich die Pharmaindustrie hauptsächlich auf die bakterielle Belastung. Für die Anwender, die unter sterilen Bedingungen arbeiten, ist die Endotoxinbelastung von speziellem Interesse. Wo auch immer die Wertigkeiten liegen, für beide Bereiche, steril wie nicht steril, sollten Partikel, Rückstandswerte sowie die mikrobiogische Belastung von Interesse sein. All das kann zu einer Kontamination des Produktes führen. Handschuhhersteller können dafür sorgen, dass die Kontaminationswerte niedrig gehalten werden: durch entsprechende Waschprozesse mit DI-Wasser, das Trocknen der Handschuhe in Hepa-gefilterten Trocknern und dass dies alles, zusammen mit dem Sortieren und Packen, im Reinraum durchgeführt wird. Alles mit dem entsprechenden Reinraum Protokoll zur Nachvollziehbarkeit der Daten und Ergebnisse. Dies wird heute bereits bei einigen Herstellern in ISO 5 oder sogar in ISO 4 Reinräumen gemacht.
Weitere Auswahlkriterien für Reinraum-Handschuhe
Komfort und persönliche Sicherheit sind für die Mitarbeiter die wichtigsten Faktoren. Ein weiterer wichtiger Faktor im Validierungsprozess ist die verfügbare Dokumentation.
Trageeigenschaften und Komfort
Gute Trageeigenschaften und Komfort sind wichtig. Wenn sich der Mitarbeiter sehr unwohl fühlt, ist es vorprogrammiert, dass Fehler passieren. Auf der anderen Seite heisst es nicht, dass ein komfortabler Handschuh automatisch die richtige Wahl ist. Naturlatex ist das meist komfortable Material, birgt aber das Risiko der Naturlatex-Protein Allergie und das Material gibt durch den hohen Abrieb sehr viele Partikel im Gebrauch an die Umgebung ab. Innenbeschichtete Handschuhe (sogenannte „gecoatete Handschuhe“) sind sicherlich sehr komfortabel für den Anwender. Die Anziehprozedur ist angenehm und schnell durch das Coating. Leider ist es sehr unwahrscheinlich, dass diese Handschuhe einen intensiven Reinigungsprozess durchlaufen – speziell mit DI-Wasser. Nachreinigung mit DI-Wasser ist aber essentiell für niedrige Rückstandswerte und Partikelwerte.
Nitrilhandschuhe bieten sicherlich nicht den Komfort, den Naturlatex Handschuhe bieten, haben aber den Vorteil, dass bei intensiver Nachreinigung mit DI-Wasser die auslösbaren Rückstände signifikant reduziert werden können. Die Abriebfestigkeit während des Tragens ist ausserdem weit geringer als bei Naturlatex Handschuhen. Die Ableitfähigkeit bei statischer Aufladung ist bei gut nachgereinigten Nitrilhandschuhen kontrollierbar – im Gegensatz zu Naturlatex. Hier würde sich eine statische Aufladung völlig unkontrolliert entladen.
Verfügbarkeit der Dokumentation
Die Möglichkeiten der Hersteller, aussagefähige und umfassende Dokumentation zur Verfügung zu stellen, sind ein Teil des Validationsprozesses. Ein weiterer Vorteil einer solch intensiven Dokumentation kann die Kostenersparnis der Anwender sein – es muss nicht jede Lot einer Prüfung unterzogen werden. Schon alleine aus diesem Grund ist eine lot-bezogene einer periodischen Dokumentation vorzuziehen. Viele periodische Inspektionen sind viertel- oder halbjährig – ein möglicherweise zu langer unkontrollierter Zeitraum. Seit vielen Jahren ist in der Elektronik-Industrie die lotbezogene Dokumentation ein Teil der „Standard Operating Procedures – SOPs“. Mehr und mehr werden diese Dokumentationen auch in der Pharmaindustrie wichtig und Teil der täglichen Routine. Folgende Daten sollten routinemäßig angefordert werden:
- Partikelangaben per Lot für sterile wie nicht sterile Handschuhe. Getestet gemäß IEST – RP – CC005.4 (2014)
- Endotoxin-Test per Lot für sterile Handschuhe – Bestätigung des sogenannten “low endotoxin claims“. Getestet gemäß dem „Limulus Amoebocyte Lysate kinetic tubidimetric” Test (LAL-Test)
- Sterilisation-Bestätigung per Lot: Gamma-sterilisiert gemäß SAL (Sterility Assurance Level) of 10⁻⁶ nach ANSI/AAMI/ EN ISO 11137:2006
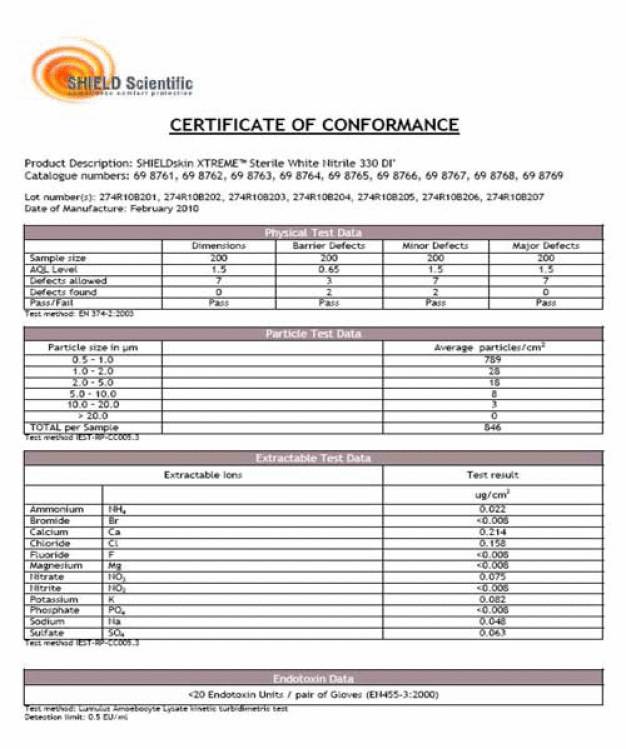
Produkt Daten Blätter, die man auf der Website der Hersteller findet, geben nur einen groben Überblick über die verfügbaren Qualitätsdaten. Um bewerten zu können, ob ein Hersteller eine kontinuierliche Dokumentation bieten kann, sollten Anwender nach Beispielen verschiedener Analysezertifikate fragen. Zertifikate über mindestens 3 Lots oder mehr geben einen guten Überblick über die Kontinuität in der Produktion. Ein Beispiel eines solchen Analysezertifikates ist angeführt. Unter Physikalischen Test Daten wird die Anzahl der Muster des fertigen Produktes, die für die physikalischen Tests verwendet wurden, angegeben. Die Spalte, die mit “barrier defects” gibt den AQL an – die statistische Ermittlung von mikroskopisch kleinen Löchern. Die Akzeptanz einer Lot hängt von der Anzahl der Defekte ab, die in diesem statistischen Test ermittelt werden (AQL – Wert). “Pass” zeigt an, ob bei dem vorgegebenen Test die Kriterien erreicht wurden. Ausserdem wird die Partikel Kontamination ausgewiesen – in “average partikel/cm² , sowie die sogenannten “extractables” – die DI-wasserlöslichen Rückstandswerte – gemessen in µg/g. (siehe Certificate of Conformance)
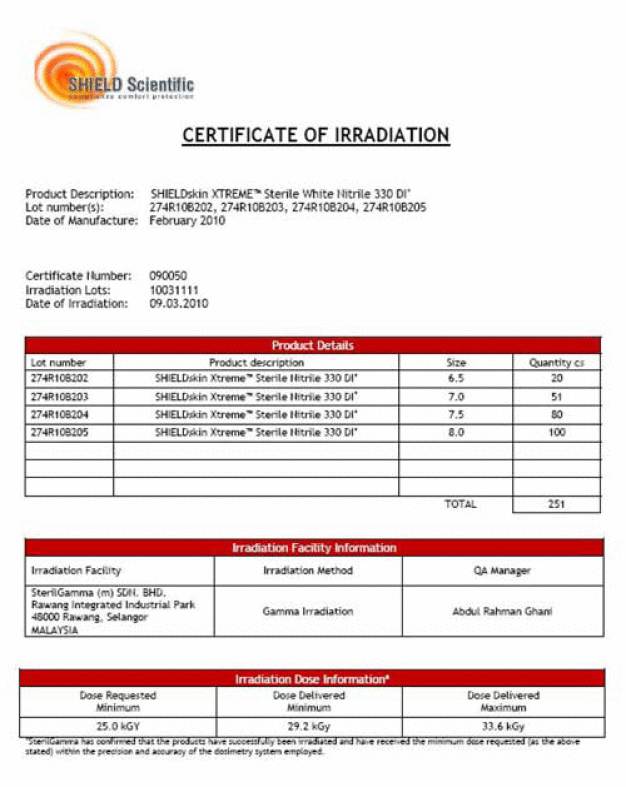
Für diejenigen, die in sterile Umgebungen arbeiten, ist es wichtig, nach einem Sterilisations-Zertifikat zu fragen, wie das nachfolgende Certificate of Irradiation. Diese Zertifikate per Lot werden oft bei GMP Audits durch die Prüfer angefordert und sind eine Grundlage bei Evaluierungsprozessen. Alle wichtigen und relevanten Informationen sind hier aufgeführt: Ort der Sterilisation, Lot Nr des zu sterilisierenden Gutes, Anzahl der Kartons, Sterilisationsart, Dosis usw. (siehe Certificate of Irradiation)
Standards und Normen
Als Teil des Risikomanagements werden Einweghandschuhe auch zum Personenschutz eingesetzt. Es ist wichtig darauf zu achten, dass die Handschuhe nach Persönlicher Schutzausrüstungs-Direktive 89/686/EEC getestet sind und als Kat III (Komplexes Design) zum Spritzschutz bei Chemikalien zertifiziert wurden wenn auf den Schutz der Person geachtet werden muss.
Handschuhe der Kat III sind für den Schutz vor Mikroorganismen und Sporen gedacht und müssen mindestens einen AQL von 1,5 Level 2) aufweisen – basierend auf dem Wasserpenetrationstest. Handschuhe dieses Typs können durchaus auch einen AQL von besser als 0,65 (Level 3) aufweisen. Der AQL (Acceptable Quality Level) ist ein wichtiger Parameter im Nachweis der Barrieren-Performanz eines Handschuhes. Bei einem statistischen Testverfahren bedeutet ein AQL von weniger als 0,65, dass die erlaubte Fehlerrate weniger als halb so hoch ist wie bei einem AQL von 1,5. Der AQL ist auch ein sehr wichtiger Faktor wenn es um den Schutz vor menschlicher Partikelkontamination geht. In den Bereichen, in denen ein hoher Schutz vor Kontamination mit Mikroorganismen und Sporen geht, sollte man auf Handschuhe mit einem AQL von 0,65 bestehen – sie bieten den höchsten Schutz vor dieser Art von Kontamination.
In Bereichen, in denen auch der Schutz vor Viren relevant ist, sollte man wissen, dass die Norm keinen Schutz vor Viren bietet. Sie ist nur für Mikroorganismen und Sporen ausgelegt. Speziell Impfstoffhersteller sind sicherlich an einem hohen Schutz vor Viren interessiert. Hier sollte entweder die Norm ASTM F1671 Virenpenetrationstest oder ISO16604:2004 angewendet werden.
Anforderung an eine spezielle Verpackung
Standardhandschuhe sind in Papierboxen verpackt und somit nicht für den Reinraum geeignet. Wenn Handschuhe so verpackt sind, neigen sie zwangsläufig dazu, Partikel, die sich von der Papierverpackung lösen, auch auf die Handschuhe und das Umfeld abzugeben. Das Herausnehmen der Handschuhe aus der Box verstärkt das Problem der Partikelabgabe. Einfach die Handschuhe in PE Folie zu verpacken statt in Boxen, löst das Problem der Partikelabgabe im Gebrauch nicht, wenn vorher keine richtige Reinigung der Handschuhe erfolgte. Grundsätzlich ist zu sagen, dass Reinraumhandschuhe nur in PE Verpackung in den Reinraum eingeschleust werden sollten. Meist sind Reinraumhandschuhe doppelt in PE verpackt für die Einschleusung. Isopropanol-resistente Tinte hilft eine weitere Kontamination zu verhindern.
In Sterilbereichen weiter mit Standard Operationshandschuhen zu arbeiten mit einer PE Aussenverpackung, jedoch einer Papierinnentasche, in die die Handschuhe gepackt sind, sind sichere Quellen für eine Kontamination. Ein weiterer Punkt ist, dass diese Handschuhe nach der Medizin Produkte Direktive (93/42/EEC) zertifiziert sind und möglicherweise von der Arbeitsschutzseite (Risiko Management – PSA) nicht die richtigen Produkte sind, um die Mitarbeiter ausreichend zu schützen.
Zusammenfassung
Um die Kontamination des Produktes und des Umfeldes unter Kontrolle zu halten ist es wichtig, möglichst nur Handschuhe einzusetzen, die mit DI-Wasser gründlich nachgereinigt, in Hepa-gefilterten Trocknern getrocknet und im Reinraum gepackt wurden (möglichst ISO Klasse 5 oder 4). Aufgeführt wurde auch, dass nicht jedes Material so ohne weiteres intensiven Waschprozessen unterzogen werden kann. Auch können nicht alle Materialien mehrfach mit DI-Wasser nachgereinigt werden. Die Konsequenz daraus ist, dass Vinyl Handschuhe möglicherweise nicht die richtige Wahl sind, wenn es um hohe Reinheit geht. In weniger kritischen Reinräumen (z.B. ISO 8 oder D – nach EC GMP Klassifikation) können möglicherweise auch kürzere Handschuhe zum Einsatz kommen 240 mm). Es ist jedoch zu bedenken, dass auch hier Probleme entstehen können durch die menschlichen Hautpartikel usw., die am Übergang von Handschuh zum Mantel/Overall frei werden. Es ist auch hier zu empfehlen auf längere Handschuhe zurück zu greifen (30 cm), um die Lücke zu schließen.
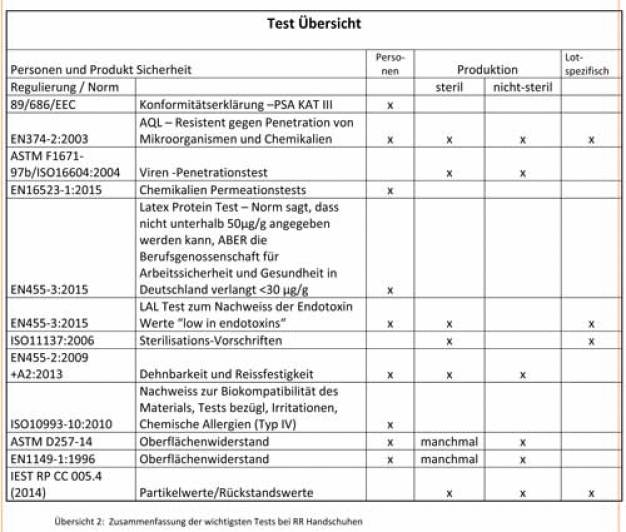
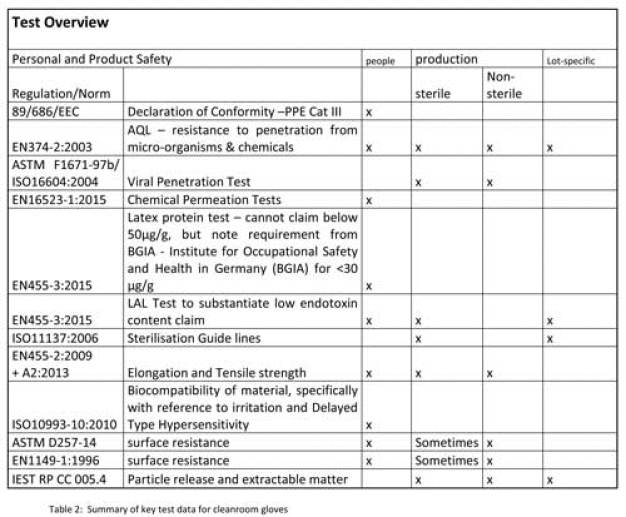
Die Diskussion der Entscheidungskriterien für Reinraum Handschuhe wurden dargelegt. Auch die Punkte Personenschutz und Produktschutz wurden ausreichend beleuchtet. Die Dokumentation ist ein entscheidendes Kriterium der langfristigen Performanz des Produktes. Als Zusammenfassung kann die Test-Übersicht für die Produktentscheidung hilfreich sein. (siehe Übersicht 2: Zusammenfassung der wichtigsten Tests bei RR Handschuhen )
References:
1) ISO 14644-1:1999 “Cleanroom and associated controlled environments – Part 1: Classification of air cleanliness”
2) VDI 2083 Part 5.1 (September 2007) “Cleanroom technology – Cleanroom operation” (VDI-Society Civil Engineering & Building Services)
3) EC Guide to Good Manufacturing Practice [Available from European Commission Enterprise Directorate General on ec.europa.eu/health/documents/eudralex/]
4) IEST-RP-CC005.3 “Gloves and Finger Cots used in Cleanroom and other Controlled Environment” (Institute of Environmental Science and Technology)
5) ISO 11137:2006 “Sterilization of health care products – radiation”
6) EN374-1:2003 “Protective gloves against chemicals and micro-organisms – Part 1: Terminology and performance requirements”
7) EN374-2:2003 “Protective gloves against chemicals and micro-organisms – Part 2: Determination of resistance to penetration”
8) EN374-3:2003 “Protective gloves against chemicals and micro-organisms – Part 3: Determination of resistance to permeation by chemicals”
9) ASTM F1671-97b /ISO16604:2004 “Standard Test Method for Resistance pf Materials Used in Protective Clothing to Penetration by Blood-borne Pathogens using Phi-X174 Bacteriophage Penetration as a Test System“
10) EN455-2:2009 + A2:2013 “Medical gloves for single-use: Part 2: Requirement and testing for physical properties”
11) EN455-3:2015 “Medical gloves for single-use: Part 3: Requirements and testing for biological evaluation”
12) ISO 10993-10 “Biological evaluation of medical devices – Part 10: Tests for irritation and delayed-type hypersenstivity“
13) ASTM D257-14 “Standard Test Methods for DC Resistance or Conductance of Insulating Materials“
14) EN1149-1:1996 “Protective clothing, electrostatic properties, surface resistivity (test methods and requirements)“
SHIELD Scientific B.V
84184 Tiefenbach
Deutschland