Particle Counting now in compliance with USP 787
Pharmaceutical procedural software PAMAS USP
The German company PAMAS develops, manufactures and distributes Automatic Particle Counters for fluid contamination control. For the analysis of pharmaceutical liquids, PAMAS offers a pharmaceutical procedural software programme, enabling the report of particle counting results in compliance with international pharmacopoeia. Before the introduction of the new feature, the programme PAMAS USP already fulfilled the standards USP 788 (Particulate Matter in Injections) and USP 789 (Particulate Matter in Ophthalmic Solutions). With a special accessory for small sample volumes, the particle counting systems PAMAS SVSS and PAMAS SBSS can now be upgraded, so as to meet the regulations of the USP 787 pharmacopoeia for Subvisible Particulate Matter in Therapeutic Protein Injections.
Excessive particulate contamination e.g. in infusion solutions or other liquid medicine may lead to health injuries. Particle analysing techniques can be used to measure particle contamination in these liquids or to make sure that pre-defined limits are not exceeded. In the pharmaceutical sector, analyses are usually done in compliance with official standards of the United States Pharmacopoeia (USP). According to the USP, pharmaceutical fluids, including infusion solutions, parenterals, pharmaceutical suspensions and intravenous or ophthalmic solutions, can be analysed with an automatic particle counter. This measuring device counts contaminants in liquids and classifies them into particle size classes.
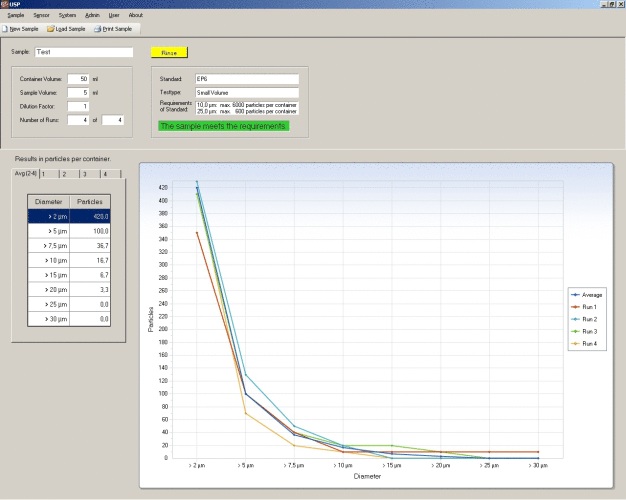
Among many other particle counter models, the PAMAS product range offers two devices that are specially designed for pharmaceutical applications: the PAMAS SVSS for the analysis of low viscous liquids and the PAMAS SBSS for high viscous samples. With these specially equipped particle analysing systems, it can be easily verified if the pharmacopoeia are being respected. The PAMAS USP pharmaceutical procedural software programme provides standardised measuring reports in compliance with the pharmacopoeia USP, EP, BP, JP and IPC. So the user can see at one glance whether the analysed sample meets the requirements of the selected standard. The PAMAS USP software can also be used to validate particle counters.
The laboratory instruments PAMAS SVSS and PAMAS SBSS are now compatible to the pharmacopoeia USP 787 (Subvisible Particulate Matter in Therapeutic Protein Injections). Up to now, it had only been possible to fulfill the pharmaceutical standards 788 (Parti¬culate Matter in Injections) and 789 (Particulate Matter in Ophthalmic Solutions). Since PAMAS has developed accessories for small sample volumes, the instruments can also fulfil the additional pharmacopoeia USP 787. Both the PAMAS SVSS for low viscous fluids and the PAMAS SBSS for high viscous fluids can be adapted to small sample volumes. The software programme PAMAS USP monitors whether the requirements of the USP 787 standard are fulfilled.
PAMAS Partikelmess- und Analysesysteme GmbH
71277 Rutesheim
Germany