A. Deal, D. Klein, P. Lopolito, und J. Schwarz von STERIS Corporation Life Sciences Division
Use of the CDC Biofilm Reactor to Test Cleaning and Disinfection on Rouged Stainless Steel
Introduction
In process systems, as in nature, microorganisms rarely exist as single cells or even as pure cultures. Rather they exist as monoculture or mixed culture aggregates of different microorganisms. Microorganisms within a biofilm, such as Pseudomonas species, are commonly encased in a slimy matrix of extracellular polymeric substances (EPS) that is important for the microorganism’s survival (1). EPS is essential to the increased tolerance to environmental stresses, antimicrobial agents and cleaning agents, an attribute associated with biofilm formation. It is critical to remove EPS prior to sanitization or disinfection.
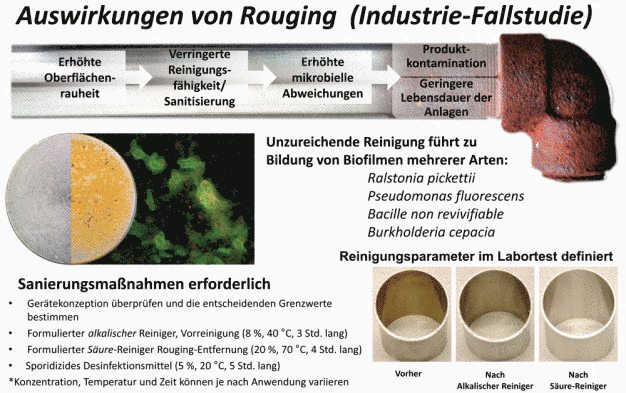
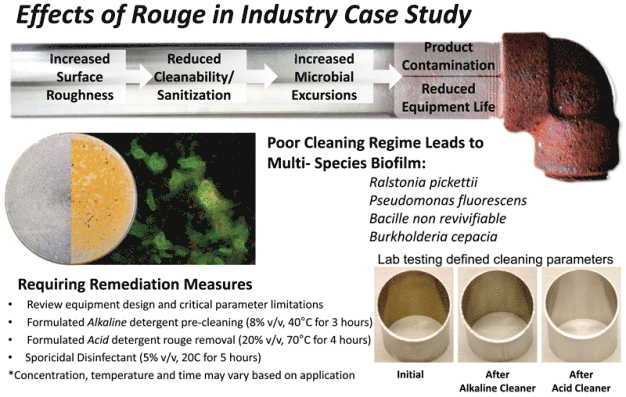
Pristine process equipment is an ideal, but rarely a reality. An iron oxide deposit known as rouge is a common occurrence in manufacturing vessels, utility lines and many other places where biofilm is found. Rouge is caused by the oxidation of steel by aqueous solutions. The presence of rouge may impact the ability of detergents and biocides to remove biofilm and disinfect equipment by increasing the surface roughness and surface area of substrates. Both of these effects may exacerbate a microbial excursion and promote the development of biofilm.
Purpose
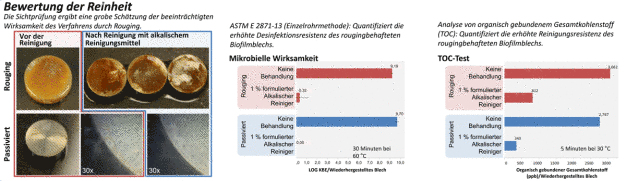
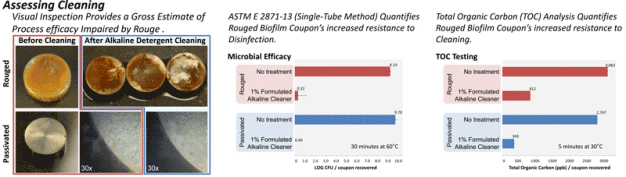
Stainless steel surfaces with the presence of rouge are a cleaning and disinfection difficulty commonly faced in process systems. Investigated in this study was the challenge to cleaning and disinfection posed by P. aeruginosa biofilm prepared on rouged stainless steel coupons in a CDC biofilm reactor system. Cleaning and disinfection using total organic carbon (TOC) surface analysis, visual cleanliness and microbial efficacy testing were evaluated.
Method
CDC Biofilm Reactor Disc Preparation:
316L stainless steel CDC discs (coupons) were passivated using a 20% v/v acidic cleaning agent at 80oC for at least 60 minutes (clean coupons). A portion of these passivated coupons were placed in an aerated sodium chloride solution containing mild carbon steel coupons for 7 days (rouged coupons). The prepared rouge can be removed with a 5% v/v acidic cleaning agent at 80°C for 10 minutes with low agitation.
Biofilm Generation:
P. aeruginosa ATCC® 15442TM was grown on R2A agar for 24 hours, transferred to tryptic soy broth (TSB) (0.3 g/L) and shaken at 130 revolutions per minute (rpm) for 24 hours at 37°C, and transferred again to TSB (0.3 g/L) in the CDC biofilm reactor assembled using the clean and rouged coupons (2, 3). The culture in the biofilm reactor was mixed at 125 rpm for 24 hours in ambient or room temperature (RT) conditions. After the initial 24 hours, the culture was perfused with fresh media (TSB 0.3 g/L ) at a rate of 11.7 mL/minute.
Microbial Efficacy:
After incubation, coupons were harvested by first dipping them in sterile de-ionized (DI) water then releasing them into a sterile petri dish. Each coupon was transferred to a 50 mL conical centrifuge tube. The ASTM single-tube method was used to challenge a formulated alkaline cleaner (1% v/v) at 60°C. Biofilm grown on either clean or rouged coupons were challenged with 4 mL of cleaning agents. To neutralize the reaction, 36 mL of cold (~4°C) letheen broth with asolectin and TWEEN® (LAT) were added to the 4 mL of formulation and vortexed. Each neutralized coupon was subjected to three cycles of 30 seconds vortexing at maximum speed and 30 seconds of sonication and sampled to obtain serial log dilutions of the neutralized reaction solution. Dilutions were pour-plated with LAT agar and incubated for 2 day at 37°C.
TOC Testing:
Cleaning with a formulated alkaline cleaner (1% v/v) was evaluated. Cleaned and rouged coupons with and without P. aeruginosa biofilm were air dried for at least 24 hours prior to cleaning (4). Treatment consisted of submerging the coupons in 1L of cleaner pre-heated to 30°C and agitated with stirring at 300 rpm. Coupons were cleaned for 5 minutes, rinsed with DI water then swabbed with low TOC polyester swabs. Swabs were then sonicated in 40 mL of DI water for 15 minutes and analyzed for TOC.
Conclusion
The CDC biofilm reactor and supporting ASTM methods provide a standardized, reproducible system for assessing the cleaning and disinfection challenge posed by biofilm. This study combined these standard methods with STERIS methodology for simulating rouged surfaces to first generate a model of rouged stainless steel and then demonstrate, qualitatively and quantitatively, that rouge can increase the cleaning and disinfection challenge posed by biofilm. Biofilm contamination is a substantial challenge to maintaining clean and disinfected processes. Rouged surfaces can accelerate surface fouling, promote biofilm development and exacerbate a microbial excursion. This synergistic relationship can quickly yield tenacious rouge/biofilm aggregates similar to those simulated in this work. The increased cleaning challenge demonstrated here highlights the need to employ effective cleaning, preventative maintenance, and disinfection strategies in a contamination control program.
References
(1) Hall-Stoodley, L. and Stoodley, P. (2002) Developmental Regulation of Microbial Biofilms. Current Opinion in Biotechnology. 13:228-233.
(2) ASTM E2562 – 12 Standard Test Method for Quantification of Pseudomonas aeruginosa Biofilm Grown with High Shear and Continuous Flow using CDC Biofilm Reactor.
(3) Buckingham-Meyer, K., Goeres, D.M. and Hamilton, M.A. (2007) Comparative evaluation of biofilm disinfectant efficacy tests. Journal of Microbiological Methods. 70: 236-244.
(4) Dell’Aringa, B., Deal, A., Klein, D., and Lopolito, P., (2013) The Use of CDC Biofilm Reactor to Test Cleaning Agents, Poster, Center for Biofilm Engineering Conference, Montana State University, Feb 5-6th, 2013.
STERIS Deutschland GmbH
50933 Köln
Germany