Jim Polarine, Jr., M.S., Carol A. Bartnett, B.S., SM (NRCM), & Marc Rogers, Ph.D.
Industry Best Practices for Disinfectant Effectiveness Testing
Abstract
Disinfectants and sporicides are critical for microbial contamination control within the pharmaceutical, biotechnology, and medical device industries. GMP manufacturing facilities are expected to demonstrate that the biocides used in controlled environments are efficacious against the facility’s environmental isolates on representative surfaces using site-specific preparation methods as described in SOPs. We present common issues highlighted by regulatory inspectors pertaining to disinfectant and sporicide qualification and potential difficulties when performing in vitro efficacy tests.
Disinfectant Qualification1
1. In vitro testing
Laboratory testing that demonstrates the effectiveness of chosen chemistries against environmental isolates on representative surfaces specific to the facility.
2. In situ testing
A statistical comparison of the frequency of isolation and the numbers of microorganisms isolated prior to and after implementation of a new disinfectant (data obtained through environmental monitoring).
Why Qualify Disinfectants?
- Regulatory expectation as part of a sound cleaning and disinfection program – “The suitability, efficacy, and limitations of disinfecting agents and procedures should be assessed”.2
- EPA registration requirements for biocidal agents do not address how they are used in the pharmaceutical, biotechnology and medical device industries.
FDA 483/Warning Letter Categories
- No data to support the appropriateness of the biocides used
- Environmental isolates not included in testing
- Coupon materials not representative of floors, walls, and work surfaces in the aseptic processing area including worn or damaged surfaces
- No data to support the expiration date of use-dilutions (e.g., hold time)
- No data to support the contact time (visible wet contact time should be achievable for the time listed in facility SOPs)
Requalification
- Review annually to assess risk and changes that have occurred
- Requalification may be necessary when:
• New disinfectants are added
• Environmental bioburden changes and/or inherently resistant organisms are isolated (e.g., B. cereus)
• New surfaces are installed
• Critical parameters in disinfectant use change
In vitro Testing
Most Common Causes for Failures
General:
- Testing biocide against inappropriate microbes
- Using inappropriate methods
- Inadequate planning
- Insufficient contact time
Neutralization:
- Inadequate neutralization
- Neutralizer toxicity
Inoculum:
- Poor viability of inoculum suspensions
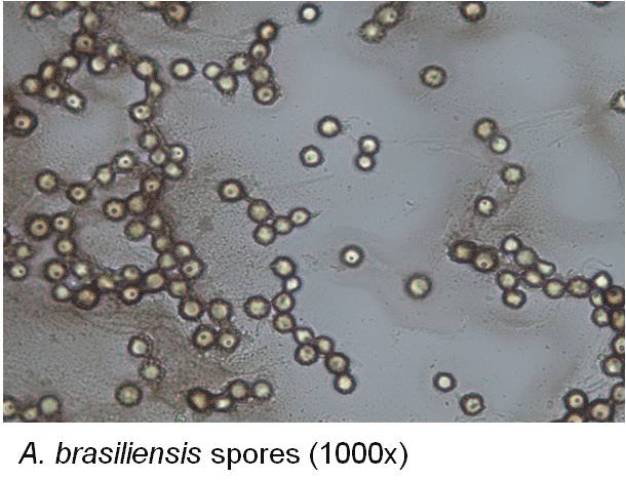
- Fungal and bacterial spore suspensions prepared incorrectly
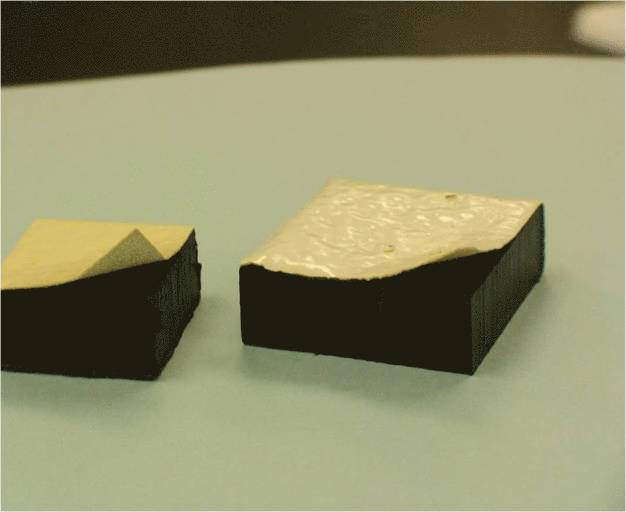
Surfaces:
- Porous surfaces
- Coupons not amenable to steam sterilization
- Uneven inoculation or product coverage due to curvature or surface tension
Recovery:
- Lethality after drying (e.g. P. aeruginosa)
- Setting artificially high log reduction targets
- Final plates are not countable
- Recovery method not validated
Key Considerations to Prevent Failures
General
- Read product labels to understand claims and limitations for each product (e.g., efficacy claims and contact times)
- Use correct chemistry type for targeted organisms (e.g., Do not test 70% IPA or quats against bacterial endospores)
- AOAC methods are inappropriate for this testing (but some procedures such as prep of inoculum and spore suspensions can be of value)
- ASTM E2197 and EN-13697 methods offer valuable insight into quantitative surface testing
- Do not combine physical removal and chemical kill in one study
- Incorporate expiry dating specified in internal SOPs into the study
- Consistency is crucial to outcome
- Upfront planning is extremely important and some experimental testing may be necessary
Neutralization
- Neutralization method must be validated
- Both neutralizer effectiveness and neutralizer toxicity must be tested
- Some trial and experimentation may be necessary prior to initiating study
- A universal neutralizer is not available for all disinfectants
Inoculum
- Prepare bacterial suspensions from 18-24 hour cultures and use immediately to inoculate surfaces; do not use over multiple days
- Use a fungal spore suspension for testing and verify spore content; hyphae/mycelia can prevent the biocide from contacting and penetrating the spores
- Prepare bacterial spore suspensions according to a recommended method such as AOAC; verify ≥90% spores
Surfaces
- Smaller coupons (1-2 cm) are easier to work with and neutralize
- Remove residues with 70% IPA
- Use alternate sterilization methods (e.g., VHP®, dry heat) if surfaces cannot be autoclaved
Recovery
- Recovery method must be validated
- Sonication may aid in recovery
- Dry inoculated coupons for shortest time necessary and test immediately
- Evaluate recovery method in experimental studies prior to initiating formal testing
Conclusion
Qualifying disinfectants is a critical step in validating the disinfection process used at a sterile manufacturing facility. Whether or not the in vitro testing is performed in-house or at a contract laboratory, avoiding the numerous issues and difficulties inherent in these studies requires thorough planning, careful attention to detail and vigilance in order to achieve a positive outcome.
References
1. USP 37 Ch Disinfectants and Antiseptics.
2. FDA Guidance for Industry,, Sterile Drug Products Produced by Aseptic Manufacturing –Current Good Manufacturing Practice. Sep. 2004. p. 34.
STERIS Deutschland GmbH
50933 Köln
Germany