Elizabeth Rivera
An "eco-friendly" assessment of cleaning agents in GMP regulated facilites
Mitigating the environmental impact of cleaning processes in GMP regulated facilities
There is a growing awareness and concern in today’s society about the “long-term maintenance of biological diversity for human well-being,” also known as environmental sustainability. This concern is growing due to a variety of factors, including global climate change, limited natural resources, human health issues, and increasing population, among others. This global issue has led to resurging demand for ‘green’ chemistry solutions for cleaning and microbial control challenges.
The origin of green chemistries
The ‘green chemistry’ concept dates back to the mid 1990’s when two chemists established 12 principles for designing chemical products and processes to reduce or eliminate the generation of hazardous waste [1]. These principles have been applied to various industrial processes including pharmaceutical, medical device and cosmetic manufacturing, and other regulated industries [2, 3]. The following discussion focuses specifically on cleaning chemistries.
The green chemistry concept focuses on the intrinsic hazard of a chemical or chemical process, and seeks to minimize that hazard to reduce personnel and environmental concerns. So, green chemistries can be viewed as risk mitigation tools.
The meaning of ‘Green’
So what exactly does ‘green cleaning’ mean? Does this term imply that the chemistry is safe for the environment; for humans and animals; in any concentration? Possibly. It may also mean that a product is made from plants and not petroleum. What about biodegradability? Recyclability?
In industry practice, ‘green’ could mean any or all of those things, and more. But a green formulation must also be effective and suitable for its intended use. A cleaning product that cannot efficiently clean (e.g. requires a high concentration, or a very long contact time to be effective) is a potential waste of resources, and is therefore the antithesis of environmentally sound.
The focus of this article is on cleaners as they relate to cleaning processes for GMP applications. A brief overview of several standards is presented to assist in understanding environmentally friendly cleaners. Next there is a discussion to answer some of the common concerns regarding current chemistries used for cleaning processing equipment in GMP regulated industries. The focus is given to relevant issues in minimizing pollution, reducing waste, managing personnel hazards, and complying with local regulations.
The current state of GMP cleaning
Cleaning procedures are required in cGMP (current Good Manufacturing Practices) industries for maintaining safe and optimally performing manufacturing equipment and facilities. The use of cleaning products to effectively remove process residues, dust, allergens, and infectious agents may be crucial to preventing product contamination that could adversely affect patient safety. But the use of cleaning products may also present health and environmental concerns. They may contain chemicals associated with skin irritation and corrosion, inhalation risks, and other human and animal health problems.
Additionally, the concentrated forms of some cleaning products are environmentally hazardous, containing ingredients that must undergo significant treatment (e.g. pH adjustment) before they can be safely discharged. Since the use of some products creates potential handling, storage, and disposal issues for users, these use factors are increasingly becoming components of the selection criteria when new or current cleaning processes are being evaluated.
Beyond consumer endorsements
Definitions of green chemistries and processes rely primarily on local legislation. However, environmental organizations, public information, and company policies regarding the environment are also influences.
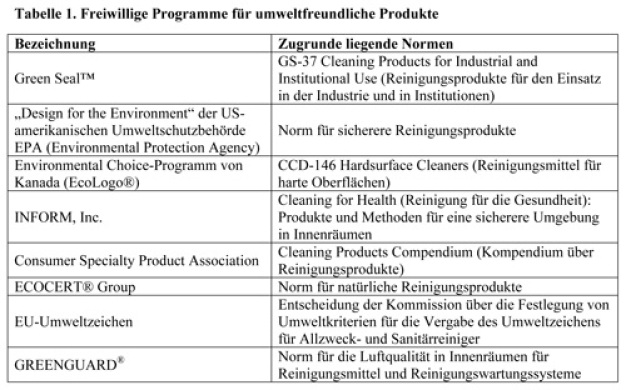
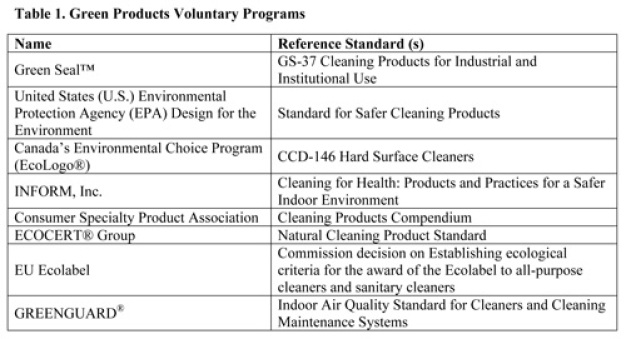
Green certification is also feasible for some categories of cleaners. Government agencies and non-profit organizations offer voluntary programs such as the U.S. EPA Design for the Environment (DfE) and the Green Seal™. These are renowned programs dedicated to the development of green products standards. However, most of these programs focus primarily on household (consumer) and janitorial type cleaning products [4 -7], which may not be optimized for use in critical GMP cleaning.
The effectiveness of cleaning procedures used in GMP regulated facilities is affected by multiple factors like temperature, action, concentration, chemistry, and contact time. Other factors affecting cleaning are soil type and conditions, type of equipment surfaces, equipment design, and others [8, 9]. Because of the many process variables and the critical nature of this cleaning, consumer-focused ‘green’ guidance may not adequately address the effectiveness of cleaning products for GMP cleaning processes.
Another potentially problematic aspect of household and janitorial products is that because of their consumer focus, the formulations are regularly changed to maintain their “New and Improved!” status in the market. This may appeal to consumers, but it presents validation nightmares for GMP cleaning.
Moreover, voluntary programs may support the use of bio-based renewable ingredients that include plant, animal, and marine mass derived materials [10, 11]. These types of ingredients may not be appropriate for GMP industries because they may pose risks associated with variable bioburden, prion contamination, and other related issues [12, 13]. The variety of manufacturing equipment, complex soils, and unique applications in these highly regulated industries makes cleaning product selection even more difficult. For these reasons, each GMP regulated site might be best served by defining their own specific ‘green’ goals.
However, some of the fundamental pollution prevention and hazard reduction principles might still be useful to GMP sites when they are developing an eco-friendly cleaning program. Table 1 provides a list of references.
This article does not intend to assess the requirements of any of the aforementioned standards or to establish criteria for green cleaning processes in the pharmaceutical and related industries. Rather this discussion addresses common issues regarding cleaning products and procedures used by GMP industry participants, and offers assistance in the selection of cleaning chemistries to ease major environmental and health concerns.
Minimizing water and air pollution
The most controversial environmental problem related to formulated detergents is the surface active agents, or surfactants [14] used as ingredients. In the European Union most of this concern has been alleviated by restricting the use of less biodegradable materials, such as tetrapropylbenzene sulfonate and certain alkyl phenol ethoxylates, through legislative ban or voluntary action. Even so, surfactants are still considered by many to be an environmental risk because they are in large-scale use by both industrial and consumer-focused manufacturers.
The overall biodegradability of surfactants has improved, in part due to legislation such as Detergent Regulation EC 648/2004, which demands stringent tests for biodegradability. This has led to the withdrawal of several surfactants from products sold in the European Union. In addition, the Organization for Economic Cooperation and Development (OECD) is developing standards for testing aquatic biodegradability of organic ingredients in products, and some have been adopted by several of the organizations listed in Table 1.
Waste water regulations are often the starting point for determining the type of cleaning agent a facility should use. Local limits may be established for some chemical species and these must be addressed before implementing a cleaning procedure. For example, the general feeling in the late 1960s was that U.S. lakes and streams were getting more polluted each day, and phosphate detergents were the primary reason. Furthermore, the presence of hypochlorite has been demonstrated to form trihalomethanes if the amount of chlorine and the nature of organic residues in the waste stream created the right conditions. Consequently, local authorities may establish limits for both phosphorus and chlorine, which necessitates limits on the amount of discharge from cleaning agents containing these materials [15, 16]. Also there may be limitations on levels of mercury, lead, cadmium, and other heavy metals.
Chelating agents may also have potential environmental drawbacks. These are substances that improve the effectiveness of formulated cleaners by preventing free metal ions in solutions from interacting with surfactants. The most frequently used chelating agents are EDTA (ethylenediaminetetraacetic acid) and NTA (nitrilotriacetic acid). The first is biodegradable (but it does so slowly) [17, 18], and the second is listed as a possible human carcinogen by the IARC (International Agency for Research on Cancer) [19].
There has also been resurging discussion about phosphates, because of the widespread use of STPP (sodium tripolyphosphate) which is a common ingredient used in the formulation of cleaning products. Since STPP is an inorganic substance, biodegradation studies are not applicable. However, STPP can be assimilated by algae and by microorganisms, and thus ends up being assimilated into the natural phosphorus cycle. Restrictions in the use of certain chelating agents are in place or are evolving in some countries. For this reason, the industry continues to search for cost-effective alternatives [20].
The pH of a formulation is another environmental factor to consider. Many municipalities have established pH restrictions in the discharge stream from industrial users. Facilities that use alkaline or acidic cleaning solutions must discharge the waste waters within acceptable discharge pH limits. If not controlled through neutralization prior to discharge, industrial users can incur substantial fines.
Cleaning agent suppliers should be able to supply charts or supplemental information to help these users establish neutralization processes. Usually, neutralization is done in specialized systems to allow mixing and temperature control. Most residues in GMP manufacturing are soluble in either a high or low pH cleaning solution. Neutralization of waste solution should not be performed in the cleaned vessel because a shift in pH may cause precipitation and re-deposition of residues onto vessel surfaces and, consequently, more cleaning steps.
Water pollution concerns are also addressed by other tests to demonstrate that substances are not toxic to aquatic life. In some U.S. states – California for example – acute aquatic toxicity would normally be determined using a fish 96-hour LC50 (lethal concentration) as required per California’s Title 22 Code of Regulations. The BOD (biological oxygen demand) and COD (chemical oxygen demand) of waste effluents are relevant in this regard. BOD is a measure of the content of biologically degradable substances in sewage while COD is commonly used to indirectly measure the amount of organic compounds in water. Generally, it is desirable to send low level BOD and COD directly to the municipal or plant wastewater system and divert high level BOD waste to a field recovery or holding tank. As in the case of LC50, BOD and COD are often governed by regulatory agencies.
Phthalates are a class of widely used industrial compounds based on esters of phthalic anhydride. There are many phthalates with many uses, and just as many toxicological properties. Phthalates are used as emulsifying agents and suspending agents in a large variety of products, from enteric coatings of pharmaceutical pills and nutritional supplements to detergents and surfactants. Despite the variety of uses, phthalates are primarily linked to plasticizers in polyvinyl chloride (PVC) piping and packaging materials. Even so, there is a growing demand for phthalate-free products after a U.S. bill was signed into law in 2008 banning the use of six types of phthalates in children’s products [21]. The ban is permanent for the use of children’s toys or childcare articles that contain more than 0.1% of di (2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP) or benzyl butyl phthalate (BBP). Environmentally conscious industries are demanding cleaning products with no hazardous phthalates and using phthalate-free PVC or stainless steel pipes in their GMP processes.
Certain VOCs (volatile organic compounds) released into the atmosphere may pose a threat to both air and water quality. VOCs are hydrocarbon compounds that have low boiling points, usually less than 100º C, and therefore they evaporate readily. Since 1990 the U.S. Clean Air Act requires abatement of a list of solvents that are hazardous air pollutants; a majority of these are toxic VOCs. Many VOCs can become a major concern for ground-water contamination because large environmental releases can be toxic to humans. Some VOC compounds can persist in ground water and migrate to drinking water supplies. Acetone, methanol, toluene, ethyl acetate and other solvents are volatile organic compounds that are used in solvent-based cleaning processes in pharmaceutical production. While not all VOCs are hazardous air pollutants, handling large quantities for cleaning processes poses a concern because the spent solvents are not easily disposed of. They require solvent recovery, treatment, or incineration. For this reason there is a growing pressure in the pharmaceutical industry to move away from VOC solvent-based cleaning to aqueous-based chemistries or less hazardous solvents.
Ease of disposal and waste reduction
The term “ease of disposal” is often applied only in reference to the cleaning agent in a GMP process; unfortunately, this is a gross oversimplification. The GMP industry deals with a wide range of process residues, including active ingredients and excipients, rouge and water scale build-up, and processing aids. Even when a cleaning agent is ‘eco-friendly,’ the spent solution may contain environmentally un-friendly residues like potent drugs and metal fines, among other things, that would not allow it to be disposed of directly into municipal sewers. Drug products manufactured at GMP sites are likely to be toxic in nature or otherwise bioactive and bioavailable (e.g. endocrine disruptors) and restrictions may be imposed on the amount of such residues that might end up in water effluents. This is where technologies like chemical and/or biological water treatment and stripping systems may need to be available on-site to ensure that waste streams meet required standards [22] .
Therefore, a spent solution’s ease of disposal would not only depend on the cleaning agents, procedures and tools that were used, but also on other residues collected in the spent solution. However, selecting a cleaning agent that poses minimal environmental impact should lessen the number of steps and resources necessary for water treatment.
Some residues may be easy to remove and some others can be tightly adhered to surfaces due to manufacturing steps that involve heat or steam. Complex residues like biopharmaceuticals may have an altered polymeric structure, which can make them more difficult to clean than their original state. Typically, parameters such as time, action, chemistry, concentration, and temperature (TACCT) determine the cleanliness achieved by a process for a specific soil or group of soils [23].
Choosing the right cleaning chemistry and parameters can help maximize productivity and reduce waste. Performing a laboratory simulation using representative materials of construction and manufacturing process soils is a good starting point to help determine the right cleaning chemistry and the optimum cleaning parameters. With this information in hand, a company can decide on the cleaning option that requires minimal raw material and utilities, and consequently produces less waste. Controlling these parameters effectively results not only in consistent cleaning performance but also reduces waste by avoiding repeated cleaning steps due to unacceptable results.
Another way of reducing waste is by recycling and reducing packaging. Most packaging of cleaning products and tools, are made of recyclable material. Plastics like HDPE (high density polyethylene), and PP (polypropylene) are mostly recommended for liquid chemistries because of their excellent chemical resistance and recyclability. Cleaning agents that are offered in bulk sizes can accommodate large, industrial consumption while also reducing the overall amount of packaging that must be dealt with. In addition, concentrated formulas can maximize the use of each unit container, which also reduces the number of empty containers.
Personnel safety management
Environmental organizations are encouraging the industries to opt for innovative systems that reduce the potential for inhalation exposure and meet other environmental goals. For example, packaging and delivery systems can be designed in such a way that they reduce operator exposure to the cleaning product.
Another great example is CIP (clean-in-place) systems, which allow for cleaning of a great deal of equipment without the added steps of dismantling it. This reduces operator exposure to potent drug residues and hazardous cleaners. It also minimizes the risk of damaging process equipment, since the assembly and disassembly of the equipment is subject to human error. If not executed correctly, it can lead to malfunction or serious damage to the equipment, and can result in spills into the environment. Moreover, CIP systems may obviate the need for personnel to get inside the vessel to clean sharp parts like agitator blades or hard-to-clean locations, and reduce the added risk of personal injury.
A cleaning process in a facility must also consider the safety of the personnel who perform the procedures and who handle the chemistries. This is of special importance in manual cleaning processes where the personnel have a higher risk of exposure. In theory, a cleaning agent used in manual applications should not contain toxic VOCs or be corrosive to skin. Unfortunately, this may not always be feasible since the majority of organic residues are most efficiently cleaned with alkaline chemistries. Therefore, if cleaning agents that are corrosive to skin or are flammable are considered, then proper PPE (personal protective equipment) and training are essential.
Regulatory compliance
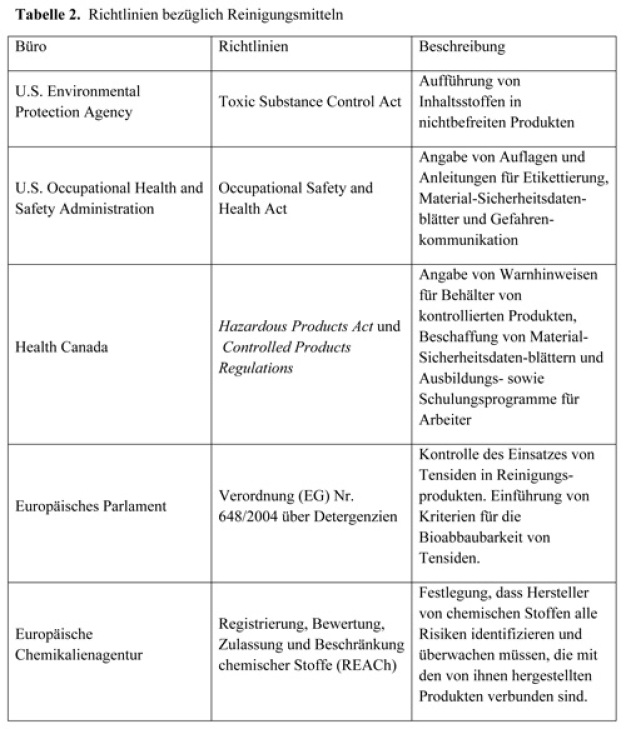
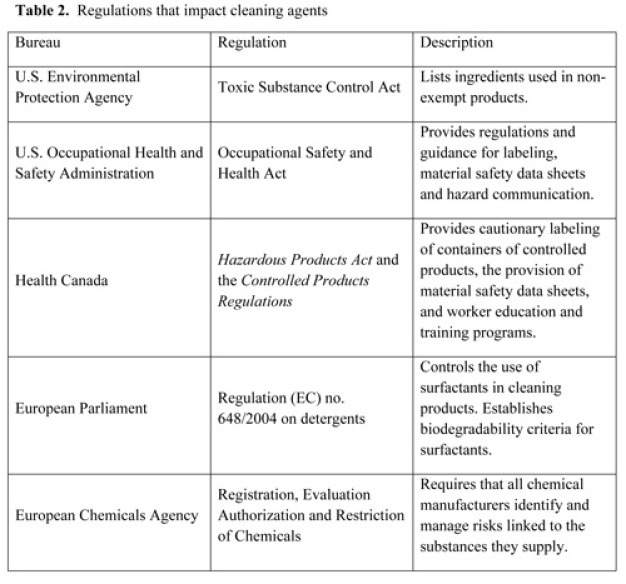
In North America and Europe, cleaning agents are regulated by one or more agencies. Each agency has an impact on the type of cleaning agents that are available and on their applications. Table 2 offers a description of some global authorities and cites their reference guidance.
Even though biocidal agents are not within the scope of this document, it is worth mentioning them in this context. From a regulatory perspective, antimicrobial agents used in GMP facilities are often considered separately from cleaning agents. Overall, there is no widely accepted definition or criteria for “environmentally preferred” antimicrobial products. For example, “non-toxic” may be an unrealistic criterion for biocidal agents since, by definition, they must be effective at killing microorganisms, especially in highly regulated environments like aseptic GMP processing areas. Environmental and health impacts can be reduced by using proper application and worker protection techniques, making appropriate choices about which antimicrobial product is necessary under what circumstances, and substituting less toxic alternatives whenever feasible.
Conclusion
In the past, the topic of ‘green’ in critical production industries has emphasized products rather than considering the whole picture, which also includes the processes. This focus on only one aspect of a complex process is not only limiting, but potentially harmful to personnel and the environment.
In the GMP industry, cleaning agents vary in type. They include formulated detergents, commodity chemicals, and solvents, and can be selected based on a variety of “green” criteria. When deciding on a cleaning process, the overall best approach takes into account performance, price, availability, regulatory requirements, and environmental impact.
As regulations continue to evolve and vary from region to region, being ‘green’ may be ‘in the eyes of the beholder.’ GMP regulated sites should evaluate, define and establish cleaning processes that best suit their individual cleaning and ‘greening’ goals.
About the Author
Elizabeth Rivera is a technical service specialist for STERIS Corporation, (Mentor, Ohio). Reach her at elizabeth_rivera@steris.com.
This article was previously published in the May/June 2013 issue of Pharmaceutical Engineering.
References
1. Anastas, P., and J. Warner. Green Chemistry: Theory and Practice. Oxford University Press. May 2000.
2. California Department of Toxic Substance Control. Green Chemistry Initiative Final Report. December 2008. pp 1-53.
3. Everts, S. Colour me green. New Scientists. March 2010. 205 (2751). pp 35-38.
4. Culver, A., et al. Cleaning for Health: Products and Practices for a Safer Indoor Environment. INFORM, Inc. 2002. pp 1 -75.
5. Green Seal, Inc. GS-37 Cleaning products for industrial and institutional use. 5th ed. August 2009. pp 1-18.
6. US EPA Design for the Environment Program. Master Criteria for Safer Ingredients. July 2010. pp 1-26.
7. US EPA Design for the Environment Program. Standard for Safer Cleaning Products. June 2009. pp 1-18.
8. Kanegsberg, B. Cleaning Agents: Overview. Handbook for Critical Cleaning- Cleaning Agents and Systems. CRC Press. 2nd ed. by B. Kanegsberg and E. Kanegsberg. 2011. pp 3-35.
9. Verghese, G., and N. Kaiser. Cleaning agents and cleaning chemistry. Cleaning and Cleaning Validation Volume I, ed. P. Pluta, published by DHI Publishing and the Parenteral Drug Association. Chapter 7. 2009. pp 103-122.
10. United States Department of Agriculture. USDA Biopreferred® Program. www.biopreferred.gov.
11. Van Engelen, G., and R. Nolles. Clean products from a clean planet for a clean future. SPFM Journal. 2010. 136 (10). pp 58-60.
12. US Food and Drug Administration. FDA Interim Final Rule, Use of Materials Derived from Cattle in Human Food and Cosmetics. July 2004.
13. European Medicines Agency. EMEA/410/01: Note For Guidance On Minimizing The Risk Of Transmitting Animal Spongiform Encephalopathy Agents Via Human And Veterinary Medicinal Products. 2011/C 73/01. Rev. 3. pp 1-18.
14. Develter, D., and L. Lauryssen. Properties and industrial applications of sophorolipids. European Journal of Lipid Science and Technology. 2010. 122 (6). pp 628-638.
15. EPA. Stage 1 Disinfectants and Disinfection Byproducts Rule (Stage 1 DBPR) 63 FR 69390. 1998. Vol. 63. No. 241.
16. EPA. Stage 2 Disinfectants and Disinfection Byproducts Rule (Stage 2 DBPR) 71 FR 388. 2006. Vol. 71. No. 2.
17. Egli, T. An aerobic breakdown of chelating agents used in household detergents. Microbiological Science. 1988. Vol 5, No 2. pp 36-41.
18. Thomas, R.A.P., et. al. Biodegradation of Metal-EDTA Complexes by an Enriched Microbial Population. Appl Environ Microbiol. 1998. April. 64 (4). pp 1319-1322.
19. International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 1999. Vol 73. pp 385-399.
20. Seetz, J., and D. Sanjose. GLDA: The new green chelating agent for detergents and cosmetics. Communications presented at the Spanish Committee of Detergency. 2008. (38). pp 33-41.
21. Govtrack.us. H.R. 4040: Consumer Product Safety Improvement Act of 2008. Title I. Section 108.
22. Martz, M. Effective Wastewater Treatment in the Pharmaceutical Industry. Pharm Eng. 2012. Nov/Dec. 32 (6). pp 48-62.
23. Verghese G., P. Lopolito. Cleaning Engineering and Equipment Design. Cleaning and Cleaning Validation Volume I, ed. P. Pluta, published by DHI Publishing and the Parenteral Drug Association. Chapter 8. 2009, pp 123-150.
STERIS Deutschland GmbH
50933 Köln
Germany