- Cleaning | procedures, devices, agents, media (Wipers, Swaps,...)
Resource-saving, reliable cleaning process for life science industries
Joint project proves suitability of quattroClean snow jet technology
The surface cleanliness required for medical and pharmaceutical products is generally achieved during manufacture using liquid-based cleaning processes that consume enormous amounts of energy and water. In order to effectively reduce resource consumption, a joint project funded by Invest BW and involving industrial partners, Fraunhofer IPA and the Natural and Medical Sciences Institute at the University of Tübingen investigated the material compatibility of the dry quattroClean snow jet cleaning technology on product surfaces typically found in the medical and pharmaceutical sectors. The results of the tests, which included in-vitro cytotoxicity tests as well as VOC and SVOC analyses, prove the suitability of the cleaning process for a wide range of applications. To lower approval hurdles, an extensive basic validation for life science applications was carried out in parallel.
In the manufacture of medical and pharmaceutical products, a cleaning process is considered suitable if contamination is reliably removed and a product-specific level of cleanliness is consistently achieved. At the same time, the surface of the product being cleaned may not be altered or damaged in any way. Traditional liquid-based cleaning processes meet these requirements for a broad range of materials used for products in the life science industry.
This level of experience is not yet widely available for the dry CO2 snow jet cleaning process “quattroClean”. The aim of the joint project involving five industrial partners, the Fraunhofer Institute for Manufacturing Engineering and Automation IPA, and the Natural and Medical Sciences Institute NMI at the University of Tübingen was therefore to demonstrate the fundamental suitability of the process for cleaning a wide variety of materials typically used in the medical and pharmaceutical industries.
Focus on surface changes and cytotoxicity
The primary aim was to prove that the mechanical forces of snow crystals do not alter, impair, or damage the surface. It was also important to establish whether the thermal stress and/or chemical properties of carbon dioxide affect the surfaces or the biocompatibility of the containers, for example by releasing cytotoxic material components.
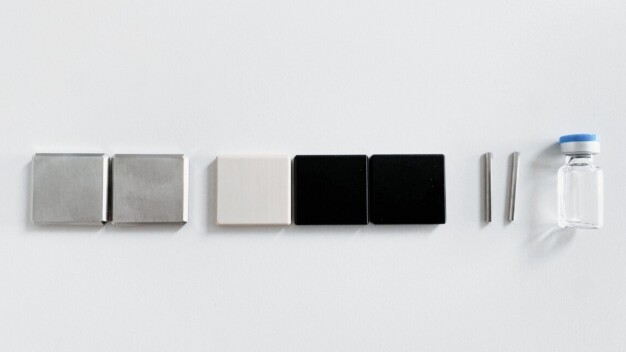
The tests were carried out on test specimens made of stainless steel 1.4301 and 1.4305 with different surface finishes, as well as polyetheretherketone (PEEK), polyether (PE), polyoxymethylene (POM), nitinol, cobalt-chromium, and glass vials.
Basic validation under worst-case conditions
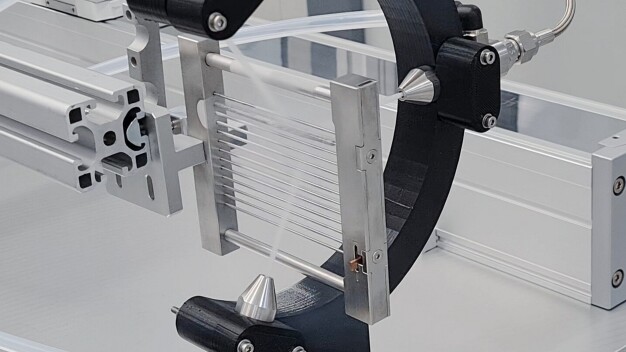
For the basic validation of the process by Fraunhofer IPA, the surfaces of the test specimens were first examined microscopically (light and/or scanning electron microscope) in their initial state. The subsequent cleaning process was carried out under worst-case conditions, i.e. the middle section and edges of the test specimens were continuously irradiated with CO2 snow at a high pressure of twelve bars for ten seconds.
Surface analysis
The ensuing surface analysis by light and scanning electron microscope showed no alterations such as structural changes, damage, changes in surface roughness, flaking, etc. on the surfaces. It was found that slightly protruding burrs on edges were partially removed.
No cracks formed in glass vials due to the cleaning process, and no propagation of existing cracks was observed. With the aid of a fluorescent penetrant, it was also possible to demonstrate that the snow crystals did not cause any additional stress in the glass. Likewise, the abrupt exposure to cold and the subsequent warming of the vials to ambient temperature did not lead to the formation of any micro cracks.
Assessment of biocompatibility
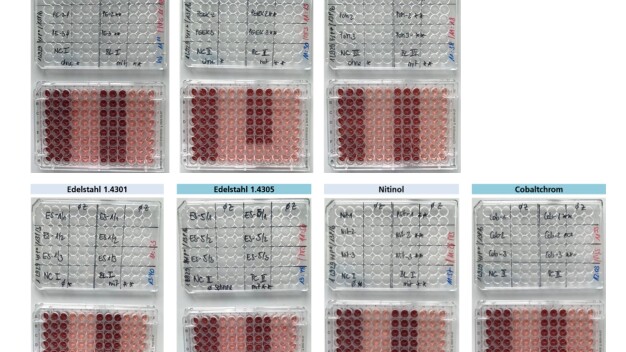
In vitro cytotoxicity tests in accordance with DIN EN ISO 10993-12: 2021-05 and DIN EN ISO 10993-12: 2021-08 confirmed that the CO2 snow does not impair cell vitality in any way. The VOC and SVOC analyses carried out in compliance with ISO 16017-1 yielded Tenax values within or below the measurement limits.
Compatibility with stainless steels
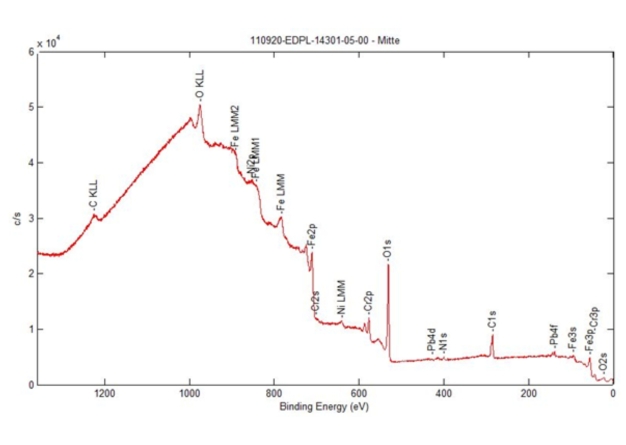
NMI tested the material compatibility of quattroClean snow jet cleaning with stainless steel 1.4301 and 1.4305 in even greater detail. In the tests, the surfaces were examined by photoelectron spectroscopy before and after cleaning with the CO2 snow jet. The comparisons and analyses showed that the process does not cause any material changes when used to clean the two types of stainless steel and can thus be classified as compatible with these materials.
Resource-saving cleaning process suitable for life science applications
Extensive testing has proven the suitability of the quattroClean snow jet technology as a resource-saving cleaning process for a wide range of applications in the medical and pharmaceutical industries. This is a dry cleaning process for full-surface and partial cleaning tasks, which uses liquid, recycled carbon dioxide as a cleaning medium. The carbon dioxide is guided through a wear-free, two-substance ring nozzle and expands on exiting to form fine snow crystals. These are bundled by a separate jacket jet of compressed air and accelerated to supersonic speed. When the easily-focused jet of snow and compressed air impacts on the surface to be cleaned, a combination of thermal, mechanical, solvent and sublimation effects occur, which is the basis of the cleaning action. With regard to residual particulate contamination, cleanliness levels in the sub-micrometer range can be reproducibly achieved. As far as filmic contamination is concerned, the cleaning result is comparable to that of other high-precision processes such as wet chemical and plasma cleaning. The removed contaminants are then extracted in the compact cleaning cell, thus preventing re-contamination of the products and contamination of the surroundings. Since the crystalline carbon dioxide sublimates completely during the process, the cleaned residue-free surfaces are dry, thereby eliminating the need for costly and energy-intensive rinsing and drying steps.
Customizable, cleanroom-compatible and integrable into production lines
To ensure that the cleaning solution is optimally adapted to the respective component requirements and geometries, as well as the production situation, the manufacturer offers a range of modular solutions and individually planned systems, including cleanroom-compatible versions for high-purity applications. These include a media preparation unit for liquid carbon dioxide, which ensures a cleanliness of 99.995 percent; the compressed air quality is 1.2.1. The process is tailored and validated according to customer and application requirements through trials in the manufacturer’s cleanroom-based technical center.
![]()
acp systems AG
Berblingerstraße 8
71254 Ditzingen
Germany
Phone: +49 7156 480140
email: info@acp-systems.com
Internet: http://acp-systems.com