- Systems
Cherwell launches Redipor® TwistLock plates to enhance sample security in microbial environmental monitoring
Cleanroom microbiology expert adds new locking lid plate options to Redipor prepared media range - available from Cherwell and AnalytiChem
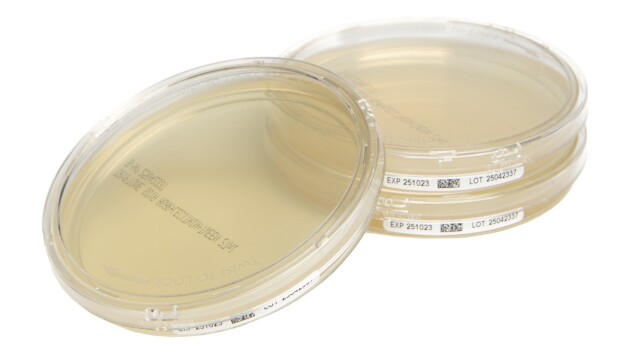
Cherwell (an Analytichem company), cleanroom microbiology solutions expert, has introduced a new range of plated prepared media designed to deliver sample security, data integrity, and optimised workflow in microbial monitoring. Cherwell’s new Redipor® TwistLock plates feature a secure locking lid and side-label with a GS1 DataMatrix barcode to enhance contamination protection, improve traceability and support automation, ensuring environmental monitoring (EM) meets exacting industry standards.
The addition of Redipor TwistLock plate options alongside standard plates in Cherwell’s broad range of Redipor prepared media products, supports a shift in pharmaceutical and associated industries towards more standardised, traceable, and risk-based microbial EM. The new locking lid plates - which are also available globally within AnalytiChem’s Redipor portfolio - align with GMP Annex 1 and ICH Q9 principles, offering benefits in terms of contamination control, regulatory compliance, and operational resilience.
Sample security is ensured by the secure seal on the plate enabled by TwistLock technology, preventing contamination and protecting sample integrity when transporting within and between facilities. Designed to deliver data integrity and reliability, Redipor TwistLock plates maintain the highest standards of data accuracy and traceability supported by GS1 barcodes on side-labels. They also support automated colony counting, plus integration with LIMS and electronic batch records. Additionally, the plates’ user-friendly design aids laboratory workflow efficiencies as they streamline handling, reduce errors and minimise costly retesting.
Like Cherwell’s standard Redipor prepared media plates, Redipor TwistLock plates are available triple-wrapped and gamma irradiated in Petri and contact plate formats to guarantee sterility and extend shelf-life to six months. Bespoke options are also available - a key offering of Cherwell’s Redipor range is the ability to customise to user needs in terms of differing media formulations, fill volumes and packaging formats.
As the UK prepared media manufacturing site for AnalytiChem, Cherwell is ensuring security of local supply to the pharmaceutical and medical manufacturing market. Expanding direct supply globally, within the Analytichem global group, Redipor TwistLock plates will also be manufactured in the U.S. and mainland Europe, in the Netherlands. This gives manufacturers choice in plated media for sampling when EM protocols may require use of locking lid plates as an alternative to standard plates.
“As a trusted supplier to the pharmaceutical and medical manufacturing industry we are always endeavouring to best understand our customers’ needs and flexibly offer the products that fit their requirements – including bespoke options,” said Yoggya De Silva, Microbiology Product Specialist, Cherwell. “Meeting Annex 1 and other key regulatory requirements by ensuring data integrity and reducing contamination risk in sterile product manufacturing processes is one such key need. The launch of our new locking lid Redipor TwistLock prepared media plates provides an answer to the demand for ever-increasing vigilance, and building resilient and reliable microbial EM programmes.”
AnalytiChem UK Ltd
OX26 4XB BICESTER
United Kingdom