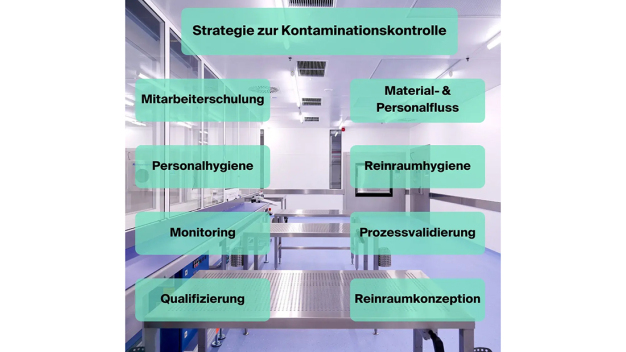
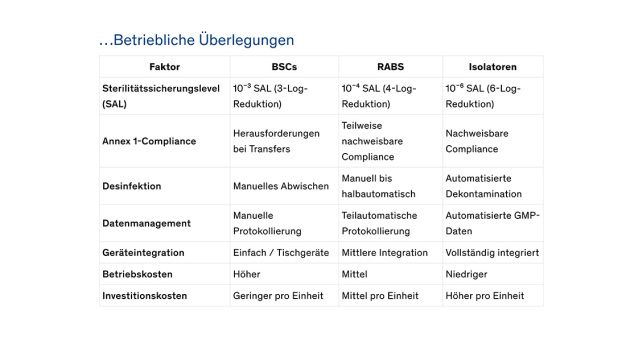
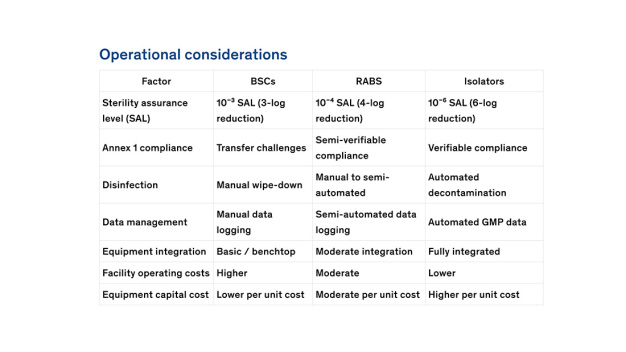
-
- ANNEX 1
Examples of sterile manufacturing and glove interventions
Annex 1 and fill-and-finish processes: possible solutions and limitations
The implementation of EU GMP Annex 1, which sets strict requirements for the production of sterile medicines, presents new challenges for pharmaceutical companies. In the foreseeable future, Ralf Wagner, Sales Director for the DACH region, Portugal and Spain at Optima Pharma, believes that glove int…