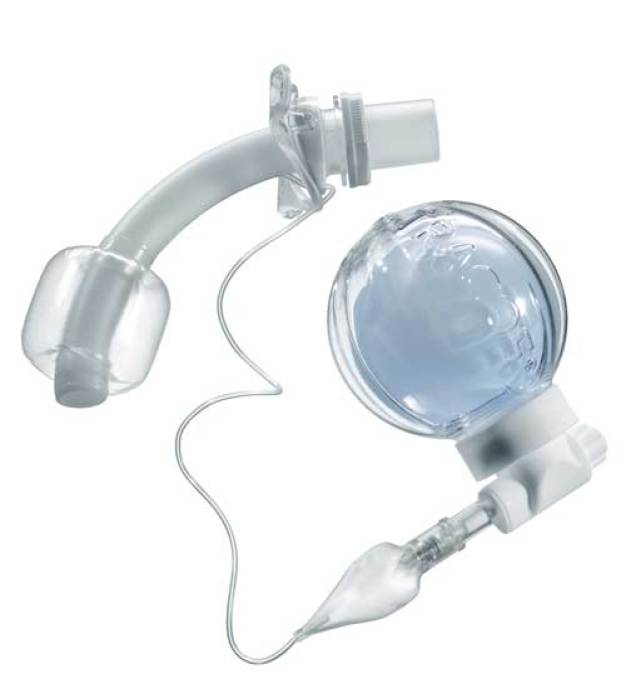
In the Green Zone – controlled ventilation of coma patients
The highest levels of safety and optimum user convenience when administering ventilation to patients is ensured with the new “smart Cuffmanager®” by Tracoe medical GmbH. This is particularly important when safeguarding the respiratory function during anaesthesia, emergency medicine and coma care, to be achieved through permanent maintenance and control of the internal pressure of the high volume low pressure cuff of the tracheostomy tube – to be in the “green zone” of between 20 and 30 cm H₂O. At the same time, very high cuff pressures and subse-quent damage to the mucous membrane are thus avoided. A special compensation balloon assures constant cuff pressure within the optimum range. As a result, the high volume low pressure cuff maintains its self-seal effect. Following 18 months’ of development and testing, introduction to the market, i.e. to hospitals, followed in mid-August 2013. At COMPAMED, experts are invited to give the Tracoe Smart Cuffmanager® a close inspection on the Spang & Brands stand – M33 – in Hall 8a.
“We have given the subject of the compensation balloon our very special attention. This system represents the central functional unit of the Smart Cuffmanager®”, according to Rd. Ralf Schnell, development manager at Tracoe medical GmbH, “ … and because of that we were looking for a plastics processing specialist with medical device competence to whom we could entrust product development and production of this sensitive product range. This task was awarded to our long-term partner Spang & Brands!” That was in the spring of 2012. At present the production process is gradually increasing.
The specialities of Spang & Brands GmbH are design optimisation of plastics components and complete systems for medical device technology and the pharmaceutical industry, the injection-moulded processing of specially formulated raw materials and multi-component injection moulding. With its entire range, from CAD-Design, tool making, to clean-room assembly and packaging of complete products, the company is well positioned to tackle all these challenges.
“For the compensation balloon we have identified a TPE material combination – ap-proved to be used in medical applications – (light blue) with an extremely low Shore A hardness (<5) in the laboratory and empirically. In addition, it is resistant to ag-gressive agents, excludes osmosis, assures repeat accuracy in long-term pressure constancy, and satisfies the strict FDA and USP requirements”, explains Friedrich Echterdiek, managing director at Spang & Brands GmbH. For the other four support components of the module ABS (white) is used, also FDA compliant. The moulds are made in-house by Spang & Brands. Due to the indentations, only collapsing core technology could be considered to achieve a secure snap connection. Thus the highly elastic TPE balloon can be connected to the housing with a snap of the rigid ABS ring. In addition, a most economical assembly process was now possible.
For the compensation balloon Spang & Brands designed and manufactured a multi- cavity mould in high chromium alloy steel in its in-house mould shop. The company also developed an innovative de-moulding technology in order to de-mould such a soft component automatically.
The required homogeneous wall thickness of the balloon body and its flange – or neck – had to be complied with extremely accurately and consistently. Therefore, the production procedure was crucial: not suitable was the process involving the customary dipping method or the blow moulding technology. Consequently, the precision injection moulding process had to be implemented. The reason: it had to be an exact reproducible component defined totally geometrically. That way the tight functional requirements could also be met.
Quality control with long-term endurance tests provides information about pressure constancy and distortions. Spang & Brands achieves the required repeatability and production safety with all-electric injection moulding machines – clamping force from 350 kN. The company produces the TPE compensation balloons as well as the support components in ABS under clean-room conditions.
Based on the entire value added chain of being a specialist in the provision of medical devices, with its more than 60 injection moulding machines of which over one third are electric, nor forgetting several multi-component injection moulding machines, Spang & Brands is able to offer the entire diversity of products optimized by plastics: “We have the right approach, supported by CAD-3D development and MoldFlow analysis”, according to Echterdiek. Medical devices are produced and packed ready to use. With our precision and cleanroom injection moulding technology the most intricate filigree components are moulded. They simply must not exceed the very tight tolerances – in the µm range. They are thus able to withstand the permanent loads to guarantee purity, hygiene and safety on and in patients. Consequently, special plastics compounds, such as TPU, TPE, TPV or Polylaktit are being used. “With multicomponent injection moulding, our approach is to combine comfort with state-of-the-art functionality. We continue to focus on TPE as an alternative to Polyisoprene. Here, many applications and new form technology are conceivable”, emphasises the Spang & Brands managing director.
Spang & Brands GmbH
61381 Friedrichsdorf
Germany